Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse
- PMID: 9444989
- PMCID: PMC124567
- DOI: 10.1128/JVI.72.2.965-974.1998
Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse
Abstract
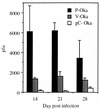
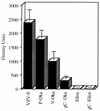
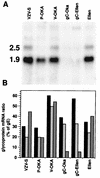
The SCID-hu mouse implanted with human fetal tissue is a novel model for investigating human viral pathogenesis. Infection of human skin implants was used to investigate the basis for the clinical attenuation of the varicella-zoster virus (VZV) strain, V-Oka, from which the newly licensed vaccine is made. The pathogenicity of V-Oka was compared with that of its parent, P-Oka, another low-passage clinical isolate, strain Schenke (VZV-S), and VZV-Ellen, a standard laboratory strain. The role of glycoprotein C (gC) in infectivity for human skin was assessed by using gC-negative mutants of V-Oka and VZV-Ellen. Whereas all of these VZV strains replicated well in tissue culture, only low-passage clinical isolates were fully virulent in skin, as shown by infectious virus yields and analysis of implant tissues for VZV DNA and viral protein synthesis. The infectivity of V-Oka in skin was impaired compared to that of P-Oka, providing the first evidence of a virologic basis for the clinical attenuation of V-Oka. The infectivity of V-Oka was further diminished in the absence of gC expression. All strains except gC-Ellen retained some capacity to replicate in human skin, but cell-free virus was recovered only from implants infected with P-Oka or VZV-S. Although VZV is closely related to herpes simplex virus type 1 (HSV-1) genetically, experiments in the SCID-hu model revealed differences in tropism for human cells that correlated with differences in VZV and HSV-1 disease. VZV caused extensive infection of epidermal and dermal skin cells, while HSV-1 produced small, superficial lesions restricted to the epidermis. As in VZV, gC expression was a determinant for viral replication in skin. VZV infects human CD4+ and CD8+ T cells in thymus/liver implants, but HSV-1 was detected only in epithelial cells, with no evidence of lymphotropism. These SCID-hu mouse experiments show that the clinical attenuation of the varicella vaccine can be attributed to decreased replication of V-Oka in skin and that tissue culture passage alone reduces the ability of VZV to infect human skin in vivo. Furthermore, gC, which is dispensable for replication in tissue culture, plays a critical role in the virulence of the human alphaherpesviruses VZV and HSV-1 for human skin.
Figures





References
-
- Aldrovandi G M, Feuer G, Gao L, Jamieson B, Kristeva M, Chen I S Y, Zack J A. The SCID-hu mouse as a model for HIV-1 infection. Nature. 1993;363:732–736. - PubMed
-
- Arvin A M. Varicella-zoster virus. In: Fields B N, Knipe D N, Howley P M, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2547–2585.
-
- Arvin A M, Koropchak C M, Williams B R G, Grumet F C, Foung S K. Early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J Infect Dis. 1986;154:422–429. - PubMed
-
- Bergen R E, Diaz P S, Arvin A M. The immunogenicity of the Oka/Merck varicella vaccine in relation to infectious varicella-zoster virus and relative viral antigen content. J Infect Dis. 1990;162:1049–1054. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

