Disruption of the G1/S transition in human papillomavirus type 16 E7-expressing human cells is associated with altered regulation of cyclin E
- PMID: 9444990
- PMCID: PMC124568
- DOI: 10.1128/JVI.72.2.975-985.1998
Disruption of the G1/S transition in human papillomavirus type 16 E7-expressing human cells is associated with altered regulation of cyclin E
Abstract
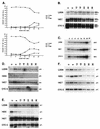
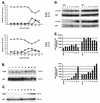
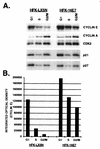
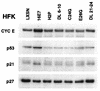
The development of neoplasia frequently involves inactivation of the p53 and retinoblastoma (Rb) tumor suppressor pathways and disruption of cell cycle checkpoints that monitor the integrity of replication and cell division. The human papillomavirus type 16 (HPV-16) oncoproteins, E6 and E7, have been shown to bind p53 and Rb, respectively. To further delineate the mechanisms by which E6 and E7 affect cell cycle control, we examined various aspects of the cell cycle machinery. The low-risk HPV-6 E6 and E7 proteins did not cause any significant change in the levels of cell cycle proteins analyzed. HPV-16 E6 resulted in very low levels of p53 and p21 and globally elevated cyclin-dependent kinase (CDK) activity. In contrast, HPV-16 E7 had a profound effect on several aspects of the cell cycle machinery. A number of cyclins and CDKs were elevated, and despite the elevation of the levels of at least two CDK inhibitors, p21 and p16, CDK activity was globally increased. Most strikingly, cyclin E expression was deregulated both transcriptionally and posttranscriptionally and persisted at high levels in S and G2/M. Transit through G1 was shortened by the premature activation of cyclin E-associated kinase activity. Elevation of cyclin E levels required both the CR1 and CR2 domains of E7. These data suggest that cyclin E may be a critical target of HPV-16 E7 in the disruption of G1/S cell cycle progression and that the ability of E7 to regulate cyclin E involves activities in addition to the release of E2F.
Figures



Similar articles
-
TGF-beta-mediated cell cycle arrest of HPV16-immortalized human ectocervical cells correlates with decreased E6/E7 mRNA and increased p53 and p21(WAF-1) expression.Exp Cell Res. 2000 Aug 25;259(1):149-57. doi: 10.1006/excr.2000.4953. Exp Cell Res. 2000. PMID: 10942587
-
Initiation of DNA synthesis by human papillomavirus E7 oncoproteins is resistant to p21-mediated inhibition of cyclin E-cdk2 activity.J Virol. 1997 Jul;71(7):5570-8. doi: 10.1128/JVI.71.7.5570-5578.1997. J Virol. 1997. PMID: 9188631 Free PMC article.
-
Analysis of the p53-mediated G1 growth arrest pathway in cells expressing the human papillomavirus type 16 E7 oncoprotein.J Virol. 1997 Apr;71(4):2905-12. doi: 10.1128/JVI.71.4.2905-2912.1997. J Virol. 1997. PMID: 9060648 Free PMC article.
-
Alteration of cell cycle in cervical tumor associated with human papillomavirus: cyclin-dependent kinase inhibitors.Yonsei Med J. 2002 Dec;43(6):722-8. doi: 10.3349/ymj.2002.43.6.722. Yonsei Med J. 2002. PMID: 12497655 Review.
-
New concepts on the role of human papillomavirus in cell cycle regulation.Ann Med. 1999 Jun;31(3):175-87. doi: 10.3109/07853899909115976. Ann Med. 1999. PMID: 10442672 Review.
Cited by
-
A dual role of cyclin E in cell proliferation and apoptosis may provide a target for cancer therapy.Curr Cancer Drug Targets. 2004 Feb;4(1):65-75. doi: 10.2174/1568009043481669. Curr Cancer Drug Targets. 2004. PMID: 14965268 Free PMC article. Review.
-
Modulation of the cell division cycle by human papillomavirus type 18 E4.J Virol. 2002 Nov;76(21):10914-20. doi: 10.1128/jvi.76.21.10914-10920.2002. J Virol. 2002. PMID: 12368334 Free PMC article.
-
Human papillomavirus type 16 E7 maintains elevated levels of the cdc25A tyrosine phosphatase during deregulation of cell cycle arrest.J Virol. 2002 Jan;76(2):619-32. doi: 10.1128/jvi.76.2.619-632.2002. J Virol. 2002. PMID: 11752153 Free PMC article.
-
Microarray analysis identifies interferon-inducible genes and Stat-1 as major transcriptional targets of human papillomavirus type 31.J Virol. 2000 May;74(9):4174-82. doi: 10.1128/jvi.74.9.4174-4182.2000. J Virol. 2000. PMID: 10756030 Free PMC article.
-
MAGT1 is required for HeLa cell proliferation through regulating p21 expression, S-phase progress, and ERK/p38 MAPK MYC axis.Cell Cycle. 2021 Nov;20(21):2233-2247. doi: 10.1080/15384101.2021.1974792. Epub 2021 Sep 9. Cell Cycle. 2021. PMID: 34499581 Free PMC article.
References
-
- Banks L M, Edmonds C, Vousden K H. Ability of the HPV16 E7 protein to bind RB and induce DNA synthesis is not sufficient for efficient transforming activity in NIH3T3 cells. Oncogene. 1990;5:1383–1389. - PubMed
-
- Blanton R A, Coltrera M D, Gown A M, Halbert C L, McDougall J K. Expression of the HPV16 E7 gene generates proliferation in stratified squamous cell cultures which is independent of endogenous p53 levels. Cell Growth Differ. 1992;3:791–802. - PubMed
-
- Boyer S N, Wazer D E, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. - PubMed
-
- Carr A M. Checkpoints take the next step. Science. 1996;271:314–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

