Construction of retroviral vectors with improved safety, gene expression, and versatility
- PMID: 9444992
- PMCID: PMC124570
- DOI: 10.1128/JVI.72.2.994-1004.1998
Construction of retroviral vectors with improved safety, gene expression, and versatility
Abstract
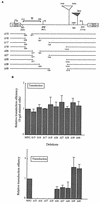
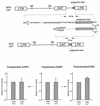
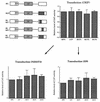
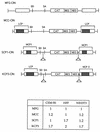
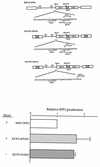
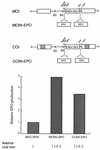
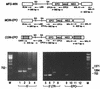
Murine leukemia virus (MLV)-based retroviral vectors are the most frequently used gene delivery vehicles. However, the current vectors are still not fully optimized for gene expression and viral titer, and many genetic and biochemical features of MLV-based vectors are poorly understood. We have previously reported that the retroviral vector MFG, where the gene of interest is expressed as a spliced mRNA, is superior in the level of gene expression with respect to other vectors compared in the study. As one approach to developing improved retroviral vectors, we have systematically performed mutational analysis of the MFG retroviral vector. We demonstrated that the entire gag coding sequence, together with the immediate upstream region, could be deleted without significantly affecting viral packaging or gene expression. To our knowledge, this region is included in all currently available retroviral vectors. In addition, almost the entire U3 region could be replaced with the heterologous human cytomegalovirus immediately-early promoter without deleterious effects. We could also insert internal ribosome entry sites (IRES) and multicloning sites into MFG without adverse effects. Based on these observations, we have constructed a series of new, improved retroviral constructs. These vectors produced viral titers comparable to MFG, expressed high levels of gene expression, and stably transferred genes to the target cells. Our vectors are more convenient to use because of the presence of multicloning sites and IRESs, and they are also more versatile because they can be readily converted to various applications. Our results have general implications regarding the design and development of improved retroviral vectors for gene therapy.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

