A spontaneous low-pathogenic variant of Theiler's virus contains an amino acid substitution within the predominant VP1(233-250) T-cell epitope
- PMID: 9444995
- PMCID: PMC124573
- DOI: 10.1128/JVI.72.2.1020-1027.1998
A spontaneous low-pathogenic variant of Theiler's virus contains an amino acid substitution within the predominant VP1(233-250) T-cell epitope
Erratum in
- J Virol 1998 Aug;72(8):6965
Abstract
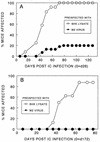
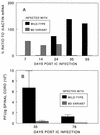
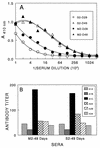
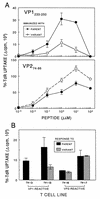
Theiler's murine encephalomyelitis virus (TMEV) induces immune-mediated demyelination after intracerebral inoculation of the virus into susceptible mouse strains. We isolated from a TMEV BeAn 8386 viral stock, a low-pathogenic variant which requires greater than a 10,000-fold increase in viral inoculation for the manifestation of detectable clinical signs. Intracerebral inoculation of this variant virus induced a strong, long-lasting, protective immunity from the demyelinating disease caused by pathogenic TMEV. The levels of antibodies to the whole virus as well as to the major linear epitopes were similar in mice infected with either the variant or wild-type virus. However, persistence of the variant virus in the central nervous system (CNS) of mice was significantly lower than that of the pathogenic virus. In addition, the T-cell response to the predominant VP1 (VP1(233-250)) epitope in mice infected with the variant virus was significantly weaker than that in mice infected with the parent virus, while similar T-cell responses were induced against another predominant epitope (VP2(74-86)). Further analyses indicated that a change of lysine to arginine at position 244 of VP1, which is the only amino acid difference in the P1 region, is responsible for such differential T-cell recognition. Thus, the difference in the T-cell reactivity to this VP1 region as well as the low level of viral persistence in the CNS may account for the low pathogenicity of this spontaneous variant virus.
Figures



References
-
- Bae Y S, Yoon J W. Determination of diabetogenicity attributable to a single amino acid, Ala776, on the polyprotein of encephalomyocarditis virus. Diabetes. 1993;42:435–443. - PubMed
-
- Chen H H, Kong W P, Zhang L, Ward P L, Roos R P. A picornaviral protein synthesized out of frame with the polyprotein plays a key role in a virus-induced immune-mediated demyelinating disease. Nat Med. 1995;1:927–931. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

