Virus-induced cell motility
- PMID: 9445023
- PMCID: PMC124601
- DOI: 10.1128/JVI.72.2.1235-1243.1998
Virus-induced cell motility
Abstract
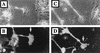
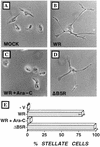
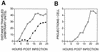
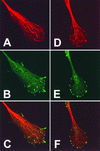
Many viruses induce profound changes in cell metabolism and function. Here we show that vaccinia virus induces two distinct forms of cell movement. Virus-induced cell migration was demonstrated by an in vitro wound healing assay in which infected cells migrated independently into the wound area while uninfected cells remained relatively static. Time-lapse microscopy showed that the maximal rate of migration occurred between 9 and 12 h postinfection. Virus-induced cell migration was inhibited by preinactivation of viral particles with trioxsalen and UV light or by the addition of cycloheximide but not by addition of cytosine arabinoside or rifampin. The expression of early viral genes is therefore necessary and sufficient to induce cell migration. Following migration, infected cells developed projections up to 160 microm in length which had growth-cone-like structures and were frequently branched. Time-lapse video microscopy showed that these projections were formed by extension and condensation of lamellipodia from the cell body. Formation of extensions was dependent on late gene expression but not the production of intracellular enveloped (IEV) particles. The requirements for virus-induced cell migration and for the formation of extensions therefore differ from each other and are distinct from the polymerization of actin tails on IEV particles. These data show that poxviruses encode genes which control different aspects of cell motility and thus represent a useful model system to study and dissect cell movement.
Figures





References
-
- Alcamí A, Smith G L. A soluble receptor for interleukin-1β encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. - PubMed
-
- Allen W E, Jones G E, Pollard J W, Ridley A J. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci. 1997;110:707–720. - PubMed
-
- Bablanian R, Baxt B, Sonnabend J A, Esteban M. Studies on the mechanisms of vaccinia virus cytopathic effects. II. Early cell rounding is associated with virus polypeptide synthesis. J Gen Virol. 1978;39:403–413. - PubMed
-
- Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel M M. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

