Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice
- PMID: 9445035
- PMCID: PMC124613
- DOI: 10.1128/JVI.72.2.1345-1353.1998
Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice
Abstract
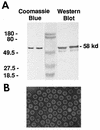
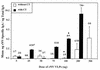
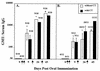
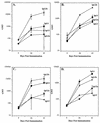
Recombinant Norwalk virus-like particles (rNV VLPs) produced in insect cells were evaluated as an oral immunogen in CD1 and BALB/c mice by monitoring rNV-specific serum total and subclass immunoglobulin G (IgG) and intestinal IgA responses. Dose and kinetics of response were evaluated in the presence and absence of the mucosal adjuvant cholera toxin (CT). rNV-specific serum IgG and intestinal IgA were detected in the absence of CT, and the number of responders was not significantly different from that of mice administered VLPs with CT at most doses. The use of CT was associated with induction of higher levels of IgG in serum; this effect was greater at higher doses of VLPs. IgG in serum was detected in the majority of animals by 9 days postimmunization (dpi), and intestinal IgA responses were detected by 24 dpi. In the absence of CT, IgG2b was the dominant IgG subclass response in both mouse strains. Thus, nonreplicating rNV VLPs are immunogenic when administered orally in the absence of any delivery system or mucosal adjuvant. These studies demonstrate that rNV VLPs are an excellent model to study the oral delivery of antigen, and they are a potential mucosal vaccine for NV infections.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

