Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor
- PMID: 9445058
- PMCID: PMC124636
- DOI: 10.1128/JVI.72.2.1542-1551.1998
Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor
Abstract
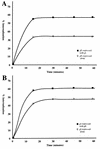
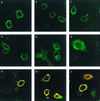
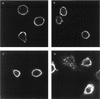
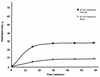
Open reading frames within the unique short segment of alphaherpesvirus genomes participate in egress and cell-to-cell spread. The case of varicella-zoster virus (VZV) is of particular interest not only because the virus is highly cell associated but also because its most prominent cell surface protein, gE, bears semblance to the mammalian Fc receptor Fc gammaRII. A previous study demonstrated that when expressed alone in cells, VZV gE was endocytosed from the cell surface through a tyrosine localization motif in its cytoplasmic tail (J. K. Olson and C. Grose, J. Virol. 71:4042-4054, 1997). Since VZV gE is normally found in association with gI in the infected cell, the present study was directed at defining the trafficking of the VZV gE:gI protein complex. First, VZV gI underwent endocytosis and recycling when it was expressed alone in cells, and interestingly, VZV gI contained a methionine-leucine internalization motif in its cytoplasmic tail. Second, VZV gI was found by confocal microscopy to colocalize with VZV gE during endocytosis and recycling in cells. Third, by a quantitative internalization assay, VZV gE:gI was shown to undergo endocytosis more efficiently (steady state, 55 to 60%) than either gE alone (steady state, approximately 32%) or gI alone (steady state, approximately 45%). Further, examination of endocytosis-deficient mutant proteins demonstrated that VZV gI exerted a more pronounced effect than gE on internalization of the complex. Most importantly, therefore, these studies suggest that VZV gI behaves as an accessory component by facilitating the endocytosis of the major constituent gE and thereby modulating the trafficking of the entire cell surface gE:gI Fc receptor complex.
Figures




Similar articles
-
Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail.J Virol. 1997 May;71(5):4042-54. doi: 10.1128/JVI.71.5.4042-4054.1997. J Virol. 1997. PMID: 9094682 Free PMC article.
-
Varicella-zoster virus glycoprotein gE: endocytosis and trafficking of the Fc receptor.J Infect Dis. 1998 Nov;178 Suppl 1:S2-6. doi: 10.1086/514255. J Infect Dis. 1998. PMID: 9852964
-
Regulation of varicella-zoster virus-induced cell-to-cell fusion by the endocytosis-competent glycoproteins gH and gE.J Virol. 2004 Mar;78(6):2884-96. doi: 10.1128/jvi.78.6.2884-2896.2004. J Virol. 2004. PMID: 14990707 Free PMC article.
-
Analysis of the functions of glycoproteins E and I and their promoters during VZV replication in vitro and in skin and T-cell xenografts in the SCID mouse model of VZV pathogenesis.Curr Top Microbiol Immunol. 2010;342:129-46. doi: 10.1007/82_2009_1. Curr Top Microbiol Immunol. 2010. PMID: 20186616 Review.
-
Herpesviral Fc receptors and their relationship to the human Fc receptors.Immunol Res. 1992;11(3-4):226-38. doi: 10.1007/BF02919129. Immunol Res. 1992. PMID: 1337547 Review.
Cited by
-
Glycoprotein E of varicella-zoster virus enhances cell-cell contact in polarized epithelial cells.J Virol. 2000 Dec;74(23):11377-87. doi: 10.1128/jvi.74.23.11377-11387.2000. J Virol. 2000. PMID: 11070038 Free PMC article.
-
Pseudorabies virus glycoprotein gD contains a functional endocytosis motif that acts in concert with an endocytosis motif in gB to drive internalization of antibody-antigen complexes from the surface of infected monocytes.J Virol. 2005 Jun;79(11):7248-54. doi: 10.1128/JVI.79.11.7248-7254.2005. J Virol. 2005. PMID: 15890963 Free PMC article.
-
Identification and Functional Characterization of a Novel Fc Gamma-Binding Glycoprotein in Rhesus Cytomegalovirus.J Virol. 2019 Feb 5;93(4):e02077-18. doi: 10.1128/JVI.02077-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30487278 Free PMC article.
-
Internalization of pseudorabies virus glycoprotein B is mediated by an interaction between the YQRL motif in its cytoplasmic domain and the clathrin-associated AP-2 adaptor complex.J Virol. 2004 Aug;78(16):8852-9. doi: 10.1128/JVI.78.16.8852-8859.2004. J Virol. 2004. PMID: 15280493 Free PMC article.
-
Promoter sequences of varicella-zoster virus glycoprotein I targeted by cellular transactivating factors Sp1 and USF determine virulence in skin and T cells in SCIDhu mice in vivo.J Virol. 2003 Jan;77(1):489-98. doi: 10.1128/jvi.77.1.489-498.2003. J Virol. 2003. PMID: 12477854 Free PMC article.
References
-
- Bremnes B, Madsen T, Gedde-Dahl M, Bakke O. An LI and ML motif in the cytoplasmic tail of the MHC-associated invariant chain mediates rapid internalization. J Cell Sci. 1994;107:2021–2032. - PubMed
-
- Collawn J F, Stangel M, Kuhn L A, Esekogwu V, Jing S, Trowbridge I S, Trainer J A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990;63:1061–1072. - PubMed
-
- Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. - PubMed
-
- De Camilli P. The eighth Datta lecture. Molecular mechanisms in synaptic vesicle recycling. FEBS Lett. 1995;369:3–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

