The spike protein of murine coronavirus mouse hepatitis virus strain A59 is not cleaved in primary glial cells and primary hepatocytes
- PMID: 9445064
- PMCID: PMC124642
- DOI: 10.1128/JVI.72.2.1606-1609.1998
The spike protein of murine coronavirus mouse hepatitis virus strain A59 is not cleaved in primary glial cells and primary hepatocytes
Abstract
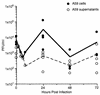
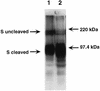
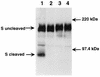
Mouse hepatitis virus strain A59 (MHV-A59) produces meningoencephalitis and severe hepatitis during acute infection. Infection of primary cells derived from the central nervous system (CNS) and liver was examined to analyze the interaction of virus with individual cell types derived from the two principal sites of viral replication in vivo. In glial cell cultures derived from C57BL/6 mice, MHV-A59 produces a productive but nonlytic infection, with no evidence of cell-to-cell fusion. In contrast, in continuously cultured cells, this virus produces a lytic infection with extensive formation of syncytia. The observation of few and delayed syncytia following MHV-A59 infection of hepatocytes more closely resembles infection of glial cells than that of continuously cultured cell lines. For MHV-A59, lack of syncytium formation correlates with lack of cleavage of the fusion glycoprotein, or spike (S) protein. The absence of cell-to-cell fusion following infection of both primary cell types prompted us to examine the cleavage of the spike protein. Cleavage of S protein was below the level of detection by Western blot analysis in MHV-A59-infected hepatocytes and glial cells. Furthermore, no cleavage of this protein was detected in liver homogenates from C57BL/6 mice infected with MHV-A59. Thus, cleavage of the spike protein does not seem to be essential for entry and spread of the virus in vivo, as well as for replication in vitro.
Figures

References
-
- Cavanagh D. The coronavirus surface glycoprotein. In: Siddell S G, editor. The Coronaviridae. New York, N.Y: Plenum Press; 1995. pp. 73–113.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

