Coadministration of DNA encoding interleukin-6 and hemagglutinin confers protection from influenza virus challenge in mice
- PMID: 9445082
- PMCID: PMC124660
- DOI: 10.1128/JVI.72.2.1704-1708.1998
Coadministration of DNA encoding interleukin-6 and hemagglutinin confers protection from influenza virus challenge in mice
Abstract
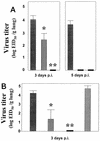
This study was conducted to investigate whether Accell gene gun coadministration of DNA encoding human interleukin-6 (IL-6) would enhance protective immune responses in mice to an equine influenza A virus hemagglutinin (HA) DNA vaccine. Mice that received HA DNA alone exhibited accelerated clearance of homologous challenge virus but were not protected from infection. In contrast, mice that received both HA and IL-6 DNA had no detectable virus in their lungs after challenge. These results strongly support the use of IL-6 as a cytokine adjuvant in DNA vaccination.
Figures


References
-
- Aarden L A, De Groot E R, Schapp O L, Lansdorp P M. Production of hybridoma growth factor by monocytes. Eur J Immunol. 1987;17:1411–1416. - PubMed
-
- Arden N H, Cox N J, Schonberger L B. Prevention and control of influenza. Morbid Mortal Weekly Rep. 1996;45:1–24.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

