Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: pivotal role of M boxes (GTCATGTGCT) and of microphthalmia
- PMID: 9447965
- PMCID: PMC108780
- DOI: 10.1128/MCB.18.2.694
Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: pivotal role of M boxes (GTCATGTGCT) and of microphthalmia
Abstract
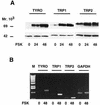
In melanocytes and in melanoma cells, cyclic AMP (cAMP)-elevating agents stimulate melanogenesis and increase the transcription of tyrosinase, the rate-limiting enzyme in melanin synthesis. However, two other enzymes, tyrosinase-related protein 1 (TRP1) and TRP2, are required for a normal melanization process leading to eumelanin synthesis. In B16 melanoma cells, we demonstrated that stimulation of melanogenesis by cAMP-elevating agents results in an increase in tyrosinase, TRP1, and TRP2 expression. cAMP, through a cAMP-dependent protein kinase pathway, stimulates TRP1 and TRP2 promoter activities in both B16 mouse melanoma cells and normal human melanocytes. Regulation of the TRP1 and TRP2 promoters by cAMP involves a M box and an E box. Further, a classical cAMP response element-like motif participates in the cAMP responsiveness of the TRP2 promoter, demonstrating that the TRP2 gene is subjected to different regulatory processes, which could account for its different expression patterns during embryonic development or under specific physiological and pathological conditions. We also found that microphthalmia, a basic helix-loop-helix transcription factor, strongly stimulates the transcriptional activities of the TRP1 and TRP2 promoters, mainly through binding to the M boxes. Additionally, we demonstrated that cAMP increases microphthalmia expression and thereby its binding to TRP1 and TRP2 M boxes. These convergent and compelling results disclose at least a part of the molecular mechanism involved in the regulation of melanogenic gene expression by cAMP and emphasize the pivotal role of microphthalmia in this process.
Figures
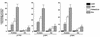



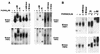

References
-
- Abdel-Malek Z, Swope V B, Amornsiripanitch N, Nordlund J J. In vitro modulation of proliferation and melanization of S91 melanoma cells by prostaglandins. Cancer Res. 1987;47:3141–3146. - PubMed
-
- Barber J I, Townsend D, Olds D P A, King R A. Dopachrome oxydoreductase: a new enzyme in the pigment pathway. J Invest Dermatol. 1984;83:145–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
