Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes
- PMID: 9447971
- PMCID: PMC108786
- DOI: 10.1128/MCB.18.2.753
Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes
Abstract
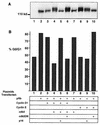
The retinoblastoma protein (pRb) acts to constrain the G1-S transition in mammalian cells. Phosphorylation of pRb in G1 inactivates its growth-inhibitory function, allowing for cell cycle progression. Although several cyclins and associated cyclin-dependent kinases (cdks) have been implicated in pRb phosphorylation, the precise mechanism by which pRb is phosphorylated in vivo remains unclear. By inhibiting selectively either cdk4/6 or cdk2, we show that endogenous D-type cyclins, acting with cdk4/6, are able to phosphorylate pRb only partially, a process that is likely to be completed by cyclin E-cdk2 complexes. Furthermore, cyclin E-cdk2 is unable to phosphorylate pRb in the absence of prior phosphorylation by cyclin D-cdk4/6 complexes. Complete phosphorylation of pRb, inactivation of E2F binding, and activation of E2F transcription occur only after sequential action of at least two distinct G1 cyclin kinase complexes.
Figures




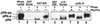
References
-
- Buchkovich K, Duffy L A, Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989;58:1097–1105. - PubMed
-
- Chellappan S P, Hiebert S, Mudryj M, Horowitz J M, Nevins J R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. - PubMed
-
- Chen P L, Scully P, Shew J Y, Wang J Y, Lee W H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989;58:1193–1198. - PubMed
-
- Chen W D, Otterson G A, Lipkowitz S, Khleif S N, Coxon A B, Kaye F J. Apoptosis is associated with cleavage of a 5 kDa fragment from RB which mimics dephosphorylation and modulates E2F binding. Oncogene. 1997;14:1243–1248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
