Characterization of a Rac1- and RhoGDI-associated lipid kinase signaling complex
- PMID: 9447972
- PMCID: PMC108787
- DOI: 10.1128/MCB.18.2.762
Characterization of a Rac1- and RhoGDI-associated lipid kinase signaling complex
Abstract
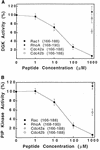
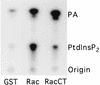
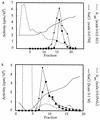
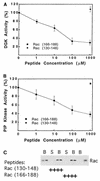
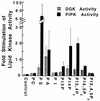
Rho family GTPases regulate a number of cellular processes, including actin cytoskeletal organization, cellular proliferation, and NADPH oxidase activation. The mechanisms by which these G proteins mediate their effects are unclear, although a number of downstream targets have been identified. The interaction of most of these target proteins with Rho GTPases is GTP dependent and requires the effector domain. The activation of the NADPH oxidase also depends on the C terminus of Rac, but no effector molecules that bind to this region have yet been identified. We previously showed that Rac interacts with a type I phosphatidylinositol-4-phosphate (PtdInsP) 5-kinase, independent of GTP. Here we report the identification of a diacylglycerol kinase (DGK) which also associates with both GTP- and GDP-bound Rac1. In vitro binding analysis using chimeric proteins, peptides, and a truncation mutant demonstrated that the C terminus of Rac is necessary and sufficient for binding to both lipid kinases. The Rac-associated PtdInsP 5-kinase and DGK copurify by liquid chromatography, suggesting that they bind as a complex to Rac. RhoGDI also associates with this lipid kinase complex both in vivo and in vitro, primarily via its interaction with Rac. The interaction between Rac and the lipid kinases was enhanced by specific phospholipids, indicating a possible mechanism of regulation in vivo. Given that the products of the PtdInsP 5-kinase and the DGK have been implicated in several Rac-regulated processes, and they bind to the Rac C terminus, these lipid kinases may play important roles in Rac activation of the NADPH oxidase, actin polymerization, and other signaling pathways.
Figures



References
-
- Abo A, Boyhan A, West I, Thrasher A J, Segal A W. Reconstitution of neutrophil NADPH oxidase activity in the cell-free system by four components: p67-phox, p47-phox, p21rac1, and cytochrome b-245. J Biol Chem. 1992;267:16767–16770. - PubMed
-
- Abo A, Pick E, Hall A, Totty N, Teahan C G, Segal A W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–670. - PubMed
-
- Ahmed S, Lee J, Kozma R, Best A, Monfries C, Lim L. A novel functional target for tumor-promoting phorbol esters and lysophosphatidic acid. The p21rac-GTPase activating protein n-chimaerin. J Biol Chem. 1993;268:10709–10712. - PubMed
-
- Bishop W R, Ganong B R, Bell R M. Attenuation of sn-1,2-diacylglycerol second messengers by diacylglycerol kinase. Inhibition by diacylglycerol analogs in vitro and in human platelets. J Biol Chem. 1986;261:6993–7000. - PubMed
-
- Bokoch G M, Bohl B P, Chuang T H. Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J Biol Chem. 1994;269:31674–31679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
