GTP hydrolysis is not important for Ypt1 GTPase function in vesicular transport
- PMID: 9447979
- PMCID: PMC108794
- DOI: 10.1128/MCB.18.2.827
GTP hydrolysis is not important for Ypt1 GTPase function in vesicular transport
Abstract
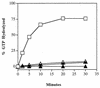
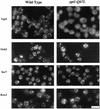
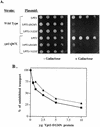
GTPases of the Ypt/Rab family play a key role in the regulation of vesicular transport. Their ability to cycle between the GTP- and the GDP-bound forms is thought to be crucial for their function. Conversion from the GTP- to the GDP-bound form is achieved by a weak endogenous GTPase activity, which can be stimulated by a GTPase-activating protein (GAP). Current models suggest that GTP hydrolysis and GAP activity are essential for vesicle fusion with the acceptor compartment or for timing membrane fusion. To test this idea, we inactivated the GTPase activity of Ypt1p by using the Q67L mutation, which targets a conserved residue that helps catalyze GTP hydrolysis in Ras. We demonstrate that the mutant Ypt1-Q67L protein is severely impaired in its ability to hydrolyze GTP both in the absence and in the presence of GAP and consequently is restricted mostly to the GTP-bound form. Surprisingly, a strain with ypt1-Q67L as the only YPT1 gene in the cell has no observable growth phenotypes at temperatures ranging from 14 to 37 degrees C. In addition, these mutant cells exhibit normal rates of secretion and normal membrane morphology as determined by electron microscopy. Furthermore, the ypt1-Q67L allele does not exhibit dominant phenotypes in cell growth and secretion when overexpressed. Together, these results lead us to suggest that, contrary to current models for Ypt/Rab function, GTP hydrolysis is not essential either for Ypt1p-mediated vesicular transport or as a timer to turn off Ypt1p-mediated membrane fusion but only for recycling of Ypt1p between compartments. Finally, the ypt1-Q67L allele, like the wild type, is inhibited by dominant nucleotide-free YPT1 mutations. Such mutations are thought to exert their dominant phenotype by sequestration of the guanine nucleotide exchange factor (GNEF). These results suggest that the function of Ypt1p in vesicular transport requires not only the GTP-bound form of the protein but also the interaction of Ypt1p with its GNEF.
Figures





References
-
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J, editors. Molecular biology of the cell. New York, N.Y: Garland Publishing, Inc.; 1994. pp. 643–644.
-
- Araki S, Kikuchi A, Hata Y, Isomura M, Takai Y. Regulation of reversible binding of smg p25A, a ras p21-like GTP-binding protein, to synaptic plasma membranes and vesicles by its specific regulatory protein, GDP dissociation inhibitor. J Biol Chem. 1990;256:13007–13015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
