Two AAA family peroxins, PpPex1p and PpPex6p, interact with each other in an ATP-dependent manner and are associated with different subcellular membranous structures distinct from peroxisomes
- PMID: 9447990
- PMCID: PMC108805
- DOI: 10.1128/MCB.18.2.936
Two AAA family peroxins, PpPex1p and PpPex6p, interact with each other in an ATP-dependent manner and are associated with different subcellular membranous structures distinct from peroxisomes
Abstract
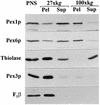
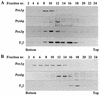
Two peroxins of the AAA family, PpPex1p and PpPex6p, are required for peroxisome biogenesis in the yeast Pichia pastoris. Cells from the corresponding deletion strains (Pp delta pex1 and Pp delta pex6) contain only small vesicular remnants of peroxisomes, the bulk of peroxisomal matrix proteins is mislocalized to the cytosol, and these cells cannot grow in peroxisome-requiring media (J. A. Heyman, E. Monosov, and S. Subramani, J. Cell Biol. 127:1259-1273, 1994; A. P. Spong and S. Subramani, J. Cell Biol. 123:535-548, 1993). We demonstrate that PpPex1p and PpPex6p interact in an ATP-dependent manner. Genetically, the interaction was observed in a suppressor screen with a strain harboring a temperature-sensitive allele of PpPEX1 and in the yeast two-hybrid system. Biochemially, these proteins were coimmunoprecipitated with antibodies raised against either of the proteins, but only in the presence of ATP. The protein complex formed under these conditions was 320 to 400 kDa in size, consistent with the formation of a heterodimeric PpPex1p-PpPex6p complex. Subcellular fractionation revealed PpPex1p and PpPex6p to be predominantly associated with membranous subcellular structures distinct from peroxisomes. Based on their behavior in subcellular fractionation experiments including flotation gradients and on the fact that these structures are also present in a Pp delta pex3 strain in which no morphologically detectable peroxisomal remnants have been observed, we propose that these structures are small vesicles. The identification of vesicle-associated peroxins is novel and implies a role for these vesicles in peroxisome biogenesis. We discuss the possible role of the ATP-dependent interaction between PpPex1p and PpPex6p in regulating peroxisome biogenesis events.
Figures






References
-
- Acharya U, Jacobs R, Peters J M, Watson N, Farquhar M G, Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995;82:895–904. - PubMed
-
- Albertini M, Rehling P, Erdmann R, Girzalsky W, Kiel J A K W, Veenhuis M, Kunau W-H. Pex14p, a peroxisomal membrane protein binding both receptors of the two PTS-dependent import pathways. Cell. 1997;89:83–92. - PubMed
-
- Arlt H, Tauer R, Feldmann H, Neupert W, Langer T. The YTA10-12 complex, an AAA protease with chaperone-like activity in the inner membrane of mitochondria. Cell. 1996;85:875–885. - PubMed
-
- Baerends R J S, Rasmussen S W, Hilbrands R E, van der Heide M, Faber K N, Reuvekamp P T W, Kiel J A K W, Cregg J M, van der Klei I J, Veenhuis M. The Hansenula polymorpha PER9 gene encodes a peroxisomal membrane protein essential for peroxisome assembly and integrity. J Biol Chem. 1996;271:8887–8894. - PubMed
-
- Beckers C J, Block M R, Glick B S, Rothman J E, Balch W E. Vesicular transport between the endoplasmic reticulum and the Golgi stack requires the NEM-sensitive fusion protein. Nature. 1989;339:397–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
