Mutation of Hip's carboxy-terminal region inhibits a transitional stage of progesterone receptor assembly
- PMID: 9447991
- PMCID: PMC108806
- DOI: 10.1128/MCB.18.2.944
Mutation of Hip's carboxy-terminal region inhibits a transitional stage of progesterone receptor assembly
Abstract
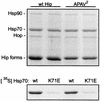
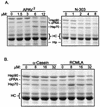
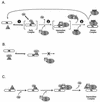
Steroid receptor complexes are assembled through an ordered, multistep pathway involving multiple components of the cytoplasmic chaperone machinery. Two of these components are Hsp70-binding proteins, Hip and Hop, that have some limited homology in their C-terminal regions, outside the sequences mapped for Hsp70 binding. Within this region of Hip is a DPEV sequence that occurs twice; in Hop, one DPEV sequence plus a partial second sequence occurs. In an effort to better understand Hip function as it relates to assembly of progesterone receptor complexes, the DPEV region of Hip was targeted for mutations. Each DPEV sequence was mutated to an APAV sequence, singly or in combination. The combined mutation, APAV2, was further combined with a deletion of Hip's tetratricopeptide repeat region that is required for Hsp70 binding or with a deletion of Hip's GGMP repeat. An additional mutant was prepared by truncation of Hip's DPEV-containing C terminus. By comparing interactions of various Hip forms with Hsp70, it was determined that mutation of the DPEV sequences created a dominant inhibitory form of Hip. The mutant Hip-Hsp70 complex was not prevented from interacting with progesterone receptor, but the mutant caused a dose-dependent inhibition of receptor assembly with Hsp90. The behavior of the Hip mutant is consistent with a model in which Hip and Hop are required to facilitate the transition from an early receptor complex with Hsp70 into later complexes containing Hsp90.
Figures






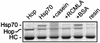
References
-
- Barent, R. L., S. C. Nair, D. C. Carr, R. A. Rimerman, Y. Ruan, Y. Zhang, J. Brennan, and D. F. Smith. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol. Endocrinol., in press. - PubMed
-
- Buchberger A, Theyssen H, Schroder H, McCarty J S, Virgallita G, Milkereit P, Reinstein J, Bukau B. Nucleotide-induced conformational changes in the ATPase and substrate binding domains of the DnaK chaperone provide evidence for interdomain communication. J Biol Chem. 1995;270:16903–16910. - PubMed
-
- Chen S, Prapapanich V, Rimerman R, Honore B, Smith D. Interactions of p60, a mediator of progesterone receptor assembly, with heat shock proteins hsp90 and hsp70. Mol Endocrinol. 1996;10:682–693. - PubMed
-
- Chen, S., V. Prapapanich, and D. F. Smith. Unpublished data.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
