Epidermal growth factor induction of the c-jun promoter by a Rac pathway
- PMID: 9448004
- PMCID: PMC108819
- DOI: 10.1128/MCB.18.2.1065
Epidermal growth factor induction of the c-jun promoter by a Rac pathway
Abstract
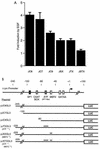
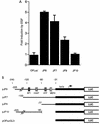
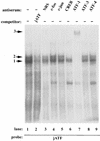
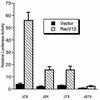
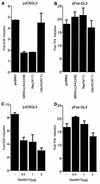
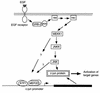
The c-jun proto-oncogene encodes a transcription factor which is activated by mitogens both transcriptionally and by phosphorylation by Jun N-terminal kinase (JNK). We have investigated the cellular signalling pathways involved in epidermal growth factor (EGF) induction of the c-jun promoter. We find that two sequence elements, which bind ATF1 and MEF2D transcription factors, are required in HeLa cells, although they are not sufficient for maximal induction. Activated forms of Ras, RacI, Cdc42Hs, and MEKK increased expression of the c-jun promoter, while dominant negative forms of Ras, RacI, and MEK kinase (MEKK) inhibited EGF induction. These and previously published results suggest that EGF activates the c-jun promoter by a Ras-to-Rac-to-MEKK pathway. This pathway is similar to that used for posttranslational activation of c-jun by JNK.
Figures


References
-
- Abate C, Luk D, Curran T. A ubiquitous nuclear protein stimulates the DNA-binding activity of fos and jun indirectly. Cell Growth Differ. 1990;1:455–462. - PubMed
-
- Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–885. - PubMed
-
- Angel P, Karin M. The role of Jun, Fos, and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. - PubMed
-
- Benbrook D M, Jones N C. Heterodimer formation between CREB and JUN proteins. Oncogene. 1990;5:295–302. - PubMed
-
- Bonni A, Ginty D D, Dudek H, Greenberg M E. Serine 133-phosphorylated CREB induces transcription via a cooperative mechanism that may confer specificity to neurotrophin signals. Mol Cell Neurosci. 1995;6:168–183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
