ATP-enhanced molecular chaperone functions of the small heat shock protein human alphaB crystallin
- PMID: 9448275
- PMCID: PMC18652
- DOI: 10.1073/pnas.95.3.1004
ATP-enhanced molecular chaperone functions of the small heat shock protein human alphaB crystallin
Abstract
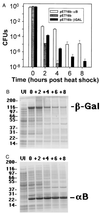
We report direct experimental evidence that human alphaB-crystallin, a member of the small heat shock protein family, actively participates in the refolding of citrate synthase (CS) in vitro. In the presence of 3.5 mM ATP, CS reactivation by alphaB-crystallin was enhanced approximately twofold. Similarly, 3.5 mM ATP enhanced the chaperone activity of alphaB-crystallin on the unfolding and aggregation of CS at 45 degrees C. Consistent with these findings, cell viability at 50 degrees C was improved nearly five orders of magnitude in Escherichia coli expressing alphaB-crystallin. SDS/PAGE analysis of cell lysates suggested that alphaB-crystallin protects cells against physiological stress in vivo by maintaining cytosolic proteins in their native and functional conformations. This report confirms the action of alphaB-crystallin as a molecular chaperone both in vitro and in vivo and describes the enhancement of alphaB-crystallin chaperone functions by ATP.
Figures



Similar articles
-
AlphaB-crystallin interacts with intermediate filaments in response to stress.J Cell Sci. 1997 Nov;110 ( Pt 21):2759-69. doi: 10.1242/jcs.110.21.2759. J Cell Sci. 1997. PMID: 9427392
-
Maintenance of chaperone-like activity despite mutations in a conserved region of murine lens alphaB crystallin.Mol Vis. 1999 Aug 10;5:15. Mol Vis. 1999. PMID: 10445957
-
Site-directed mutations within the core "alpha-crystallin" domain of the small heat-shock protein, human alphaB-crystallin, decrease molecular chaperone functions.J Mol Biol. 1999 Jun 4;289(2):397-411. doi: 10.1006/jmbi.1999.2759. J Mol Biol. 1999. PMID: 10366513
-
Substrate Interaction Networks of the Escherichia coli Chaperones: Trigger Factor, DnaK and GroEL.Adv Exp Med Biol. 2015;883:271-94. doi: 10.1007/978-3-319-23603-2_15. Adv Exp Med Biol. 2015. PMID: 26621473 Review.
-
Proteostasis and the Regulation of Intra- and Extracellular Protein Aggregation by ATP-Independent Molecular Chaperones: Lens α-Crystallins and Milk Caseins.Acc Chem Res. 2018 Mar 20;51(3):745-752. doi: 10.1021/acs.accounts.7b00250. Epub 2018 Feb 14. Acc Chem Res. 2018. PMID: 29442498 Review.
Cited by
-
Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance.Planta. 2003 Nov;218(1):1-14. doi: 10.1007/s00425-003-1105-5. Epub 2003 Sep 26. Planta. 2003. PMID: 14513379 Review.
-
alpha-crystallin assists the renaturation of glyceraldehyde-3-phosphate dehydrogenase.Biochem J. 2000 Feb 1;345 Pt 3(Pt 3):467-72. Biochem J. 2000. PMID: 10642503 Free PMC article.
-
Heterologous expression of a plant small heat-shock protein enhances Escherichia coli viability under heat and cold stress.Plant Physiol. 1999 Jun;120(2):521-8. doi: 10.1104/pp.120.2.521. Plant Physiol. 1999. PMID: 10364403 Free PMC article.
-
Oligomeric structure and chaperone-like activity of Drosophila melanogaster mitochondrial small heat shock protein Hsp22 and arginine mutants in the alpha-crystallin domain.Cell Stress Chaperones. 2017 Jul;22(4):577-588. doi: 10.1007/s12192-017-0784-y. Epub 2017 Apr 7. Cell Stress Chaperones. 2017. PMID: 28389817 Free PMC article.
-
Small heat shock proteins from extremophiles: a review.Extremophiles. 2004 Feb;8(1):1-11. doi: 10.1007/s00792-003-0362-3. Epub 2003 Nov 19. Extremophiles. 2004. PMID: 15064984 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

