Human erythropoietin dimers with markedly enhanced in vivo activity
- PMID: 9448306
- PMCID: PMC18713
- DOI: 10.1073/pnas.95.3.1184
Human erythropoietin dimers with markedly enhanced in vivo activity
Abstract
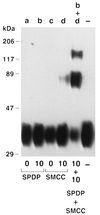
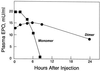
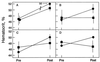
Human erythropoietin, a widely used and important therapeutic glycoprotein, has a relatively short plasma half-life due to clearance by glomerular filtration as well as by other mechanisms. We hypothesized that an erythropoietin species with a larger molecular size would exhibit an increased plasma half-life and, potentially, an enhanced biological activity. We now report the production of biologically active erythropoietin dimers and trimers by chemical crosslinking of the conventional monomeric form. We imparted free sulfhydryl residues to a pool of erythropoietin monomer by chemical modification. A second pool was reacted with another modifying reagent to yield monomer with maleimido groups. Upon mixing these two pools, covalently linked dimers and trimers were formed that were biologically active in vitro. The plasma half-life of erythropoietin dimers in rabbits was >24 h compared with 4 h for the monomers. Importantly, erythropoietin dimers were biologically active in vivo as shown by their ability to increase the hematocrits of mice when injected subcutaneously. In addition, the dimers exhibited >26-fold higher activity in vivo than did the monomers and were very effective after only one dose. Dimeric and other oligomeric forms of Epo may have an important role in therapy.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

