Antibacterial efficacy against an in vivo Salmonella typhimurium infection model and pharmacokinetics of a liposomal ciprofloxacin formulation
- PMID: 9449259
- PMCID: PMC105454
- DOI: 10.1128/AAC.42.1.45
Antibacterial efficacy against an in vivo Salmonella typhimurium infection model and pharmacokinetics of a liposomal ciprofloxacin formulation
Abstract
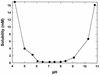
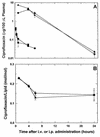
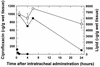
The fluoroquinolone antibiotic ciprofloxacin has been encapsulated into large unilamellar vesicles (LUV) at efficiencies approaching 100%. Drug accumulation proceeded in response to a transmembrane gradient of methylammonium sulfate and occurred concomitantly with the efflux of methylamine. A mechanism for the encapsulation process is described. LUV composed of dipalmitoylphosphatidylcholine-cholesterol (DPPC/chol), distearoylphosphatidylcholine-cholesterol (DSPC/chol), or sphingomyelin-cholesterol (SM/chol) increased the circulation lifetime of ciprofloxacin after intravenous (i.v.) administration by > 15-fold. The retention of ciprofloxacin in liposomes in the circulation decreased in the sequence SM/chol > DSPC/chol > DPPC/chol. Increased circulation lifetimes were associated with enhanced delivery of the drug to the livers, spleens, kidneys, and lungs of mice. Encapsulation of ciprofloxacin also conferred significant increases in the longevity of the drug in the plasma after intraperitoneal administration and in the lungs after intratracheal administration in comparison to free ciprofloxacin. The efficacy of a single i.v. administration of an SM/chol formulation of ciprofloxacin was measured in a Salmonella typhimurium infection model. At 20 mg of ciprofloxacin per kg of body weight, the encapsulated formulation resulted in 10(3)- to 10(4)-fold fewer viable bacteria in the livers and spleens of infected mice than was observed for animals treated with free ciprofloxacin. These results show the utility of liposomal encapsulation of ciprofloxacin in improving the pharmacokinetics, biodistribution, and antibacterial efficacy of the antibiotic. In addition, these formulations are well suited for i.v., intraperitoneal, and intratracheal or aerosol administration.
Figures





References
-
- Bermudez L E, Yao-Young A O, Lin J-P, Cogger J, Young L S. Treatment of disseminated Mycobacterium avium complex infection of beige mice with liposome-encapsulated aminoglycosides. J Infect Dis. 1990;161:1262–1268. - PubMed
-
- Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. - PubMed
-
- Boman N L, Bally M B, Cullis P R, Mayer L D, Webb M S. Encapsulation of vincristine in liposomes reduces its toxicity and improves its anti-tumor efficacy. J Liposome Res. 1995;5:523–541.
-
- Boman N L, Masin D, Mayer L D, Cullis P R, Bally M B. Liposomal vincristine which exhibits increased drug retention and increased circulation longevity cures mice bearing P388 tumors. Cancer Res. 1994;54:2830–2833. - PubMed
-
- Cullis P R, Hope M J, Bally M B, Madden T D, Mayer L D, Fenske D B. Influence of pH gradients on the transbilayer transport of drugs, lipids, peptides and metal ions into large unilamellar vesicles. Biochim Biophys Acta. 1997;1331:187–211. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

