Dicaffeoylquinic and dicaffeoyltartaric acids are selective inhibitors of human immunodeficiency virus type 1 integrase
- PMID: 9449274
- PMCID: PMC105469
- DOI: 10.1128/AAC.42.1.140
Dicaffeoylquinic and dicaffeoyltartaric acids are selective inhibitors of human immunodeficiency virus type 1 integrase
Abstract
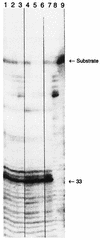
Current pharmacological agents for human immunodeficiency virus (HIV) infection include drugs targeted against HIV reverse transcriptase and HIV protease. An understudied therapeutic target is HIV integrase, an essential enzyme that mediates integration of the HIV genome into the host chromosome. The dicaffeoylquinic acids (DCQAs) and the dicaffeoyltartaric acids (DCTAs) have potent activity against HIV integrase in vitro and prevent HIV replication in tissue culture. However, their specificity against HIV integrase in cell culture has been questioned. Thus, the ability of the DCQAs and DCTAs to inhibit binding of HIV type 1 (HIV-1) gp120 to CD4 and their activities against HIV-1 reverse transcriptase and HIV RNase H were studied. The DCQAs and DCTAs inhibited HIV-1 integrase at concentrations between 150 and 840 nM. They inhibited HIV replication at concentrations between 2 and 12 microM. Their activity against reverse transcriptase ranged from 7 microM to greater than 100 microM. Concentrations that inhibited gp120 binding to CD4 exceeded 80 microM. None of the compounds blocked HIV-1 RNase H by 50% at concentrations exceeding 80 microM. Furthermore, when the effects of the DCTAs on reverse transcription in acutely infected cells were measured, they were found to have no activity. Therefore, the DCQAs and DCTAs exhibit > 10- to > 100-fold specificity for HIV integrase, and their activity against integrase in biochemical assays is consistent with their observed anti-HIV activity in tissue culture. Thus, the DCQAs and DCTAs are a potentially important class of HIV inhibitors that act at a site distinct from that of current HIV therapeutic agents.
Figures
References
-
- Aboulker J, Swart A M. Preliminary analysis of the Concorde trial. Lancet. 1993;341:889–890. - PubMed
-
- Baldwin E T, Bhat T N, Liu B, Pattabiraman N, Erickson J W. Structural basis of drug resistance for the V82A mutant of HIV-1 proteinase. Nat Struct Biol. 1995;2:244–249. - PubMed
-
- Brinkworth R I, Fairlie D P. Non-peptidic anti-AIDS agents: inhibition of HIV-1 proteinase by disulfonates. Biochem Biophys Res Commun. 1992;188:624–630. - PubMed
-
- Brinkworth R I, Woon T C, Fairlie D P. Inhibition of HIV-1 proteinase by non-peptide carboxylates. Biochem Biophys Res Commun. 1991;176:241–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

