The lantibiotic mersacidin inhibits peptidoglycan synthesis by targeting lipid II
- PMID: 9449277
- PMCID: PMC105472
- DOI: 10.1128/AAC.42.1.154
The lantibiotic mersacidin inhibits peptidoglycan synthesis by targeting lipid II
Abstract
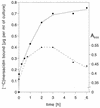
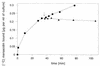
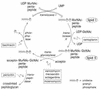
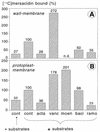
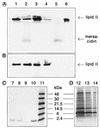
The lantibiotic mersacidin exerts its bactericidal action by inhibition of peptidoglycan biosynthesis. It interferes with the membrane-associated transglycosylation reaction; during this step the ultimate monomeric peptidoglycan precursor, undecaprenyl-pyrophosphoryl-MurNAc-(pentapeptide)-GlcNAc (lipid II) is converted into polymeric nascent peptidoglycan. In the present study we demonstrate that the molecular basis of this inhibition is the interaction of mersacidin with lipid II. The adsorption of [14C]mersacidin to growing cells, as well as to isolated membranes capable of in vitro peptidoglycan synthesis, was strictly dependent on the availability of lipid II, and antibiotic inhibitors of lipid II formation strongly interfered with this binding. Direct evidence for the interaction was provided by studies with isolated lipid II. [14C]mersacidin associated tightly with [14C]lipid II micelles; the complex was stable even in the presence of 1% sodium dodecyl sulfate. Furthermore, the addition of isolated lipid II to the culture broth efficiently antagonized the bactericidal activity of mersacidin. In contrast to the glycopeptide antibiotics, complex formation does not involve the C-terminal D-alanyl-D-alanine moiety of the lipid intermediate. Thus, the interaction of mersacidin with lipid II apparently occurs via a binding site which is not targeted by any antibiotic currently in use.
Figures
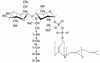
References
-
- Anderson J S, Matsuhashi M, Haskin M A, Strominger J L. Biosynthesis of the peptidoglycan of bacterial cell walls. II. Phospholipid carriers in the reaction sequence. J Biol Chem. 1967;242:3180–3190. - PubMed
-
- Barrett M S, Wenzel R P, Jones R N. In vitro activity of mersacidin (M87-1551), an investigational peptide antibiotic tested against gram-positive bloodstream isolates. Diagn Microbiol Infect Dis. 1992;15:641–644. - PubMed
-
- Best G K, Durham N N. Vancomycin adsorption to Bacillus subtilis cell walls. Arch Biochem Biophys. 1965;111:685–692. - PubMed
-
- Bierbaum G, Brötz H, Koller K-P, Sahl H-G. Cloning, sequencing and production of the lantibiotic mersacidin. FEMS Lett. 1995;127:121–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

