Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPIbalpha
- PMID: 9449713
- PMCID: PMC2212123
- DOI: 10.1084/jem.187.3.329
Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPIbalpha
Abstract
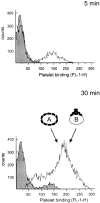
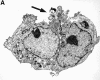
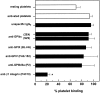
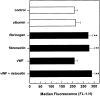
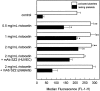
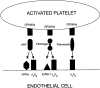
Although it has been reported that activated platelets can adhere to intact endothelium, the receptors involved have not been fully characterized. Also, it is not clear whether activated platelets bind primarily to matrix proteins at sites of endothelial cell denudation or directly to endothelial cells. Thus, this study was designed to further clarify the mechanisms of activated platelet adhesion to endothelium. Unstimulated human umbilical vein endothelial cell (HUVEC) monolayers were incubated with washed, stained, and thrombin-activated human platelets. To exclude matrix involvement, HUVEC were harvested mechanically and platelet binding was measured by flow cytometry. Before the adhesion assay, platelets or HUVEC were treated with different receptor antagonists. Whereas blockade of platelet beta1 integrins, GPIbalpha, GPIV, P-selectin, and platelet-endothelial cell adhesion molecule (PECAM)-1 did not reduce platelet adhesion to HUVEC, blockade of platelet GPIIbIIIa by antibodies or Arg-Gly-Asp (RGD) peptides markedly decreased adhesion. Moreover, when platelets were treated with blocking antibodies to GPIIbIIIa-binding adhesive proteins, including fibrinogen and fibronectin, and von Willebrand factor (vWF), platelet binding was also reduced markedly. Addition of fibrinogen, fibronectin, or vWF further increased platelet adhesion, indicating that both endogenous platelet-exposed and exogenous adhesive proteins can participate in the binding process. Evaluation of the HUVEC receptors revealed predominant involvement of intercellular adhesion molecule (ICAM)-1 and alphavbeta3 integrin. Blockade of these two receptors by antibodies decreased platelet binding significantly. Also, there was evidence that a component of platelet adhesion was mediated by endothelial GPIbalpha. Blockade of beta1 integrins, E-selectin, P-selectin, PECAM-1, vascular cell adhesion molecule (VCAM)-1 and different matrix proteins on HUVEC did not affect platelet adhesion. In conclusion, we show that activated platelet binding to HUVEC monolayers is mediated by a GPIIbIIIa-dependent bridging mechanism involving platelet-bound adhesive proteins and the endothelial cell receptors ICAM-1, alphavbeta3 integrin, and, to a lesser extent, GPIbalpha.
Figures





References
-
- Abrams C, Shattil SJ. Immunological detection of activated platelets in clinical disorders. Thromb Haemostasis. 1991;65:467–473. - PubMed
-
- Fry GL, Czervionke RL, Hoak JC, Smith JB, Haycraft DL. Platelet adherence to cultured vascular cells: influence of prostacyclin (PGI2) Blood. 1980;55:271–275. - PubMed
-
- Kaplan JE, Moon DG, Weston LK, Minnear FL, Del Vecchio PJ, Shepard JM, Fenton JW. Platelets adhere to thrombin-treated endothelial cells in vitro. Am J Physiol. 1989;257:H423–H433. - PubMed
-
- Tloti MA, Moon DG, Weston LK, Kaplan JE. Effect of 13-hydroxy octadeca-9,11-dienoic acid (13-HODE) on thrombin induced platelet adherence to endothelial cells in vitro. Thromb Res. 1991;62:305–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

