Selective induction of apoptosis in mature T lymphocytes by variant T cell receptor ligands
- PMID: 9449715
- PMCID: PMC2212120
- DOI: 10.1084/jem.187.3.349
Selective induction of apoptosis in mature T lymphocytes by variant T cell receptor ligands
Abstract
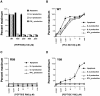
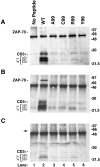
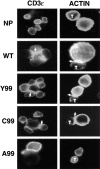
Activation, anergy, and apoptosis are all possible outcomes of T cell receptor (TCR) engagement. The first leads to proliferation and effector function, whereas the others can lead to partial or complete immunological tolerance. Structural variants of immunizing peptide-major histocompatibility complex molecule ligands that induce selective lymphokine secretion or anergy in mature T cells in association with altered intracellular signaling events have been described. Here we describe altered ligands for mature mouse CD4(+) T helper 1 cells that lead to T cell apoptosis by the selective expression of Fas ligand (FasL) and tumor necrosis factor (TNF) without concomitant IL-2, IL-3, or interferon gamma production. All ligands that stimulated cell death were found to induce FasL and TNF mRNA expression and TCR aggregation ("capping") at the cell surface, but did not elicit a common pattern of tyrosine phosphorylation of the TCR-associated signal transduction chains. Thus, TCR ligands that uniquely trigger T cell apoptosis without inducing cytokines that are normally associated with activation can be identified.
Figures

Similar articles
-
Threshold signaling of human Th0 cells in activation and anergy: modulation of effector function by altered TCR ligand.J Immunol. 2000 Jun 1;164(11):6034-40. doi: 10.4049/jimmunol.164.11.6034. J Immunol. 2000. PMID: 10820288
-
Partial signaling by CD8+ T cells in response to antagonist ligands.J Exp Med. 1996 Jul 1;184(1):149-57. doi: 10.1084/jem.184.1.149. J Exp Med. 1996. PMID: 8691128 Free PMC article.
-
Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists.Science. 1995 Jan 27;267(5197):515-8. doi: 10.1126/science.7824949. Science. 1995. PMID: 7824949
-
Suboptimal engagement of the T-cell receptor by a variety of peptide-MHC ligands triggers T-cell anergy.Immunology. 2010 Jan;129(1):1-7. doi: 10.1111/j.1365-2567.2009.03206.x. Epub 2009 Dec 2. Immunology. 2010. PMID: 20002785 Free PMC article. Review.
-
Variant TCR ligands: new insights into the molecular basis of antigen-dependent signal transduction and T-cell activation.Semin Immunol. 1996 Apr;8(2):83-101. doi: 10.1006/smim.1996.0011. Semin Immunol. 1996. PMID: 8920243 Review.
Cited by
-
Harnessing programmed cell death as a therapeutic strategy in rheumatic diseases.Nat Rev Rheumatol. 2011 Mar;7(3):152-60. doi: 10.1038/nrrheum.2010.225. Epub 2011 Feb 1. Nat Rev Rheumatol. 2011. PMID: 21283145 Free PMC article. Review.
-
Peptide-based immunotherapy of autoimmunity: a path of puzzles, paradoxes and possibilities.Immunology. 2001 Dec;104(4):367-76. doi: 10.1046/j.1365-2567.2001.01324.x. Immunology. 2001. PMID: 11899421 Free PMC article. Review. No abstract available.
-
Deficiency of small GTPase Rac2 affects T cell activation.J Exp Med. 2001 Oct 1;194(7):915-26. doi: 10.1084/jem.194.7.915. J Exp Med. 2001. PMID: 11581314 Free PMC article.
-
Restimulation-induced apoptosis of T cells is impaired in patients with X-linked lymphoproliferative disease caused by SAP deficiency.J Clin Invest. 2009 Oct;119(10):2976-89. doi: 10.1172/JCI39518. Epub 2009 Sep 14. J Clin Invest. 2009. PMID: 19759517 Free PMC article.
-
Creating therapeutic cancer vaccines: notes from the battlefield.Trends Immunol. 2001 Jan;22(1):5-7. doi: 10.1016/s1471-4906(00)01793-2. Trends Immunol. 2001. PMID: 11286676 Free PMC article.
References
-
- Evavold BD, Sloan LJ, Allen PM. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol Today. 1993;14:602–609. - PubMed
-
- Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-zeta and lack of ZAP-70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. - PubMed
-
- Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995;267:515–518. - PubMed
-
- Jameson SC, Bevan MJ. T cell receptor antagonists and partial agonists. Immunity. 1995;2:1–11. - PubMed
-
- Madrenas J, Germain RN. Variant TCR ligands: new insights into the molecular basis of antigen-dependent signal transduction and T cell activation. Semin Immunol. 1996;8:83–101. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

