Manipulation of catalase levels produces altered photosynthesis in transgenic tobacco plants
- PMID: 9449845
- PMCID: PMC35165
- DOI: 10.1104/pp.116.1.259
Manipulation of catalase levels produces altered photosynthesis in transgenic tobacco plants
Erratum in
- Plant Physiol 1998 Feb;116(2):870
Abstract
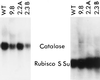
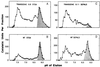
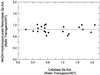
Constructs containing the cDNAs encoding the primary leaf catalase in Nicotiana or subunit 1 of cottonseed (Gossypium hirsutum) catalase were introduced in the sense and antisense orientation into the Nicotiana tabacum genome. The N. tabacum leaf cDNA specifically overexpressed CAT-1, the high catalatic [corrected] form, activity. Antisense constructs reduced leaf catalase specific activities from 0.20 to 0.75 times those of wild type (WT), and overexpression constructs increased catalase specific activities from 1.25 to more than 2.0 times those of WT. The NADH-hydroxypyruvate reductase specific activity in transgenic plants was similar to that in WT. The effect of antisense constructs on photorespiration was studied in transgenic plants by measuring the CO2 compensation point (gamma) at a leaf temperature of 38 degrees C. A significant linear increase was observed in gamma with decreasing catalase (at 50% lower catalase activity gamma increased 39%). There was a significant temperature-dependent linear decrease in gamma in transgenic leaves with elevated catalase compared with WT leaves (at 50% higher catalase gamma decreased 17%). At 29 degrees C, gamma also decreased with increasing catalase in transgenic leaves compared with WT leaves, but the trend was not statistically significant. Rates of dark respiration were the same in WT and transgenic leaves. Thus, photorespiratory losses of CO2 were significantly reduced with increasing catalase activities at 38 degrees C, indicating that the stoichiometry of photorespiratory CO2 formation per glycolate oxidized normally increases at higher temperatures because of enhanced peroxidation.
Figures


References
-
- Chamnongpol S, Willekens H, Langebartels C, Van Montagu M, Inze D, Van Camp W. Transgenic tobacco with a reduced catalase activity develops necrotic lesions and induces pathogenesis-related expression under high light. Plant J. 1996;10:491–503.
-
- Chen Z, Green D, Westhoff C, Spreitzer RJ. Nuclear mutation restores the reduced CO2/O2 specificity of ribulose bisphosphate carboxylase/oxygenase in a temperature-conditional chloroplast mutant of Chlamydomonas reinhardtii. Arch Biochem Biophys. 1990;283:60–67. - PubMed
-
- Comai L, Moran P, Maslyar D. Novel and useful properties of a chimeric plant promoter combining CaMV 35S and MAS elements. Plant Mol Biol. 1990;15:373–381. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

