Nucleotide triphosphates are required for the transport of glycolate oxidase into peroxisomes
- PMID: 9449847
- PMCID: PMC35171
- DOI: 10.1104/pp.116.1.309
Nucleotide triphosphates are required for the transport of glycolate oxidase into peroxisomes
Abstract
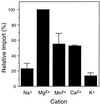
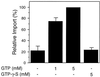
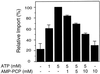
All peroxisomal proteins are nuclear encoded, synthesized on free cytosolic ribosomes, and posttranslationally targeted to the organelle. We have used an in vitro assay to reconstitute protein import into pumpkin (Cucurbita pepo) glyoxysomes, a class of peroxisome found in the cotyledons of oilseed plants, to study the mechanisms involved in protein transport across peroxisome membranes. Results indicate that ATP hydrolysis is required for protein import into peroxisomes; nonhydrolyzable analogs of ATP could not substitute for this requirement. Nucleotide competition studies suggest that there may be a nucleotide binding site on a component of the translocation machinery. Peroxisomal protein import also was supported by GTP hydrolysis. Nonhydrolyzable analogs of GTP did not substitute in this process. Experiments to determine the cation specificity of the nucleotide requirement show that the Mg2+ salt was preferred over other divalent and monovalent cations. The role of a putative protonmotive force across the peroxisomal membrane was also examined. Although low concentrations of ionophores had no effect on protein import, relatively high concentrations of all ionophores tested consistently reduced the level of protein import by approximately 50%. This result suggests that a protonmotive force is not absolutely required for peroxisomal protein import.
Figures




References
-
- Balch WE. Small GTP-binding proteins in vesicular transport. Trends Biochem Sci. 1990;15:473–477. - PubMed
-
- Bellion E, Goodman JM. Proton ionophores prevent assembly of a peroxisomal protein. Cell. 1987;48:165–173. - PubMed
-
- Cline K, Ettinger WF, Theg SM. Protein-specific energy requirements for protein transport across or into thylakoid membranes. J Biol Chem. 1992;267:2688–2696. - PubMed
-
- del Valle R, Soto U, Necochea C, Leighton F. Detection of an ATPase activity in rat liver peroxisomes. Biochem Biophys Res Commun. 1988;156:1353–1359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

