Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression
- PMID: 9450925
- PMCID: PMC316485
- DOI: 10.1101/gad.12.3.290
Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression
Abstract
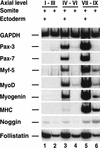
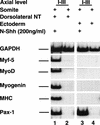
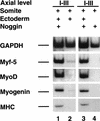
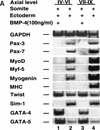
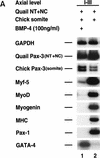
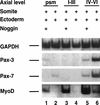
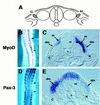
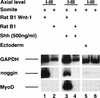
Previous work has indicated that signals from the neural tube, notochord, and surface ectoderm promote somitic myogenesis. Here, we show that somitic myogenesis is under negative regulation as well; BMP signaling serves to inhibit the activation of MyoD and Myf5 in Pax3-expressing cells. Furthermore, we show that the BMP antagonist Noggin is expressed within the dorsomedial lip of the dermomyotome, where Pax3-expressing cells first initiate the expression of MyoD and Myf5 to give rise to myotomal cells in the medial somite. Consistent with the expression of Noggin in dorsomedial dermomyotomal cells that lie adjacent to the dorsal neural tube, we have found that coculture of somites with fibroblasts programmed to secrete Wnt1, which is expressed in dorsal neural tube, can induce somitic Noggin expression. Ectopic expression of Noggin lateral to the somite dramatically expands MyoD expression into the lateral regions of the somite, represses Pax3 expression in this tissue, and induces formation of a lateral myotome. Together, our findings indicate that the timing and location of myogenesis within the somite is controlled by relative levels of BMP activity and localized expression of a BMP antagonist.
Figures





Similar articles
-
Compartmentalization of the somite and myogenesis in chick embryos are influenced by wnt expression.Dev Biol. 2000 Dec 1;228(1):86-94. doi: 10.1006/dbio.2000.9921. Dev Biol. 2000. PMID: 11087628
-
Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue.Cell. 1997 Apr 4;89(1):139-48. doi: 10.1016/s0092-8674(00)80190-7. Cell. 1997. PMID: 9094722
-
Control of dorsoventral somite patterning by Wnt-1 and beta-catenin.Dev Biol. 1998 Jan 15;193(2):182-94. doi: 10.1006/dbio.1997.8806. Dev Biol. 1998. PMID: 9473323
-
Wnt signaling and the activation of myogenesis in mammals.EMBO J. 1999 Dec 15;18(24):6867-72. doi: 10.1093/emboj/18.24.6867. EMBO J. 1999. PMID: 10601008 Free PMC article. Review.
-
Role of growth factors in shaping the developing somite.Mol Cell Endocrinol. 1998 May 25;140(1-2):83-7. doi: 10.1016/s0303-7207(98)00033-1. Mol Cell Endocrinol. 1998. PMID: 9722173 Review.
Cited by
-
Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro.Nat Protoc. 2016 Oct;11(10):1833-50. doi: 10.1038/nprot.2016.110. Epub 2016 Sep 1. Nat Protoc. 2016. PMID: 27583644
-
BMP antagonists enhance myogenic differentiation and ameliorate the dystrophic phenotype in a DMD mouse model.Neurobiol Dis. 2011 Feb;41(2):353-60. doi: 10.1016/j.nbd.2010.10.003. Epub 2010 Oct 16. Neurobiol Dis. 2011. PMID: 20940052 Free PMC article.
-
Spatiotemporal expression of Wnt5a during the development of the striated muscle complex in rats with anorectal malformations.Int J Clin Exp Pathol. 2014 Apr 15;7(5):1997-2005. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 24966909 Free PMC article.
-
Obesity Resistance and Enhanced Insulin Sensitivity in Ahnak-/- Mice Fed a High Fat Diet Are Related to Impaired Adipogenesis and Increased Energy Expenditure.PLoS One. 2015 Oct 14;10(10):e0139720. doi: 10.1371/journal.pone.0139720. eCollection 2015. PLoS One. 2015. PMID: 26466345 Free PMC article.
-
Identification of satellite cells from anole lizard skeletal muscle and demonstration of expanded musculoskeletal potential.Dev Biol. 2018 Jan 15;433(2):344-356. doi: 10.1016/j.ydbio.2017.08.037. Epub 2017 Dec 25. Dev Biol. 2018. PMID: 29291980 Free PMC article.
References
-
- Amthor H, Connolly D, Patel K, Brand-Saberi B, Wilkinson DG, Cooke J, Christ B. The expression and regulation of follistatin and a follistatin-like gene during avian somite compartmentalization and myogenesis. Dev Biol. 1996;178:343–362. - PubMed
-
- Avery G, Chow M, Holtzer H. An experimental analysis of the development of the spinal column. J Exp Zool. 1956;132:409–425.
-
- Barth J, Ivarie R. Polyvinyl alcohol enhances detection of low abundance transcripts in early stage quail embryos in a nonradioactive whole mount in situ hybridization technique. Biotechniques. 1994;17:324–327. - PubMed
-
- Basler K, Edlund T, Jessel T, Yamada T. Control of cell pattern in the neural tube: Regulation of cell differentiation by dorsalin-1, a novel TGF-β family member. Cell. 1993;73:687–702. - PubMed
-
- Buckingham M. Making muscle in mammals. Trends Genet. 1992;8:144–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
