Activation of Shaker potassium channels. II. Kinetics of the V2 mutant channel
- PMID: 9450945
- PMCID: PMC2222768
- DOI: 10.1085/jgp.111.2.295
Activation of Shaker potassium channels. II. Kinetics of the V2 mutant channel
Abstract
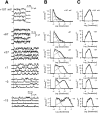
This second of three papers, in which we functionally characterize activation gating in Shaker potassium channels, focuses on the properties of a mutant channel (called V2), in which the leucine at position 382 (in the Shaker B sequence) is mutated to valine. The general properties of V2's ionic and gating currents are consistent with changes in late gating transitions, in particular, with V2 disrupting the positively cooperative gating process of the normally activating wild type (WT) channel. An analysis of forward and backward rate constants, analogous to that used for WT in the previous paper, indicates that V2 causes little change in the rates for most of the transitions in the activation path, but causes large changes in the backward rates of the final two transitions. Single channel data indicate that the V2 mutation causes moderate changes in the rates of transitions to states that are not in the activation path, but little change in the rates from these states. V2's data also yield insights into the general properties of the activation gating process that could not be readily obtained from the WT channel, including evidence that intermediate transitions have rapid backward rates, and an estimate of a total charge 2 e0 for the final two transitions. Taken together, these data will help constrain an activation gating model in the third paper of this series, while also providing an explanation for V2's effects.
Figures





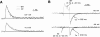

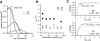

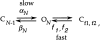
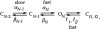
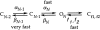
Similar articles
-
Activation of Shaker potassium channels. III. An activation gating model for wild-type and V2 mutant channels.J Gen Physiol. 1998 Feb;111(2):313-42. doi: 10.1085/jgp.111.2.313. J Gen Physiol. 1998. PMID: 9450946 Free PMC article.
-
Activation of shaker potassium channels. I. Characterization of voltage-dependent transitions.J Gen Physiol. 1998 Feb;111(2):271-94. doi: 10.1085/jgp.111.2.271. J Gen Physiol. 1998. PMID: 9450944 Free PMC article.
-
Determinants of voltage-dependent gating and open-state stability in the S5 segment of Shaker potassium channels.J Gen Physiol. 1999 Aug;114(2):215-42. doi: 10.1085/jgp.114.2.215. J Gen Physiol. 1999. PMID: 10435999 Free PMC article.
-
Mutations in the S4 region isolate the final voltage-dependent cooperative step in potassium channel activation.J Gen Physiol. 1999 Mar;113(3):389-414. doi: 10.1085/jgp.113.3.389. J Gen Physiol. 1999. PMID: 10051516 Free PMC article.
-
Gating of voltage-dependent potassium channels.Prog Biophys Mol Biol. 2001;75(3):165-99. doi: 10.1016/s0079-6107(01)00006-2. Prog Biophys Mol Biol. 2001. PMID: 11376798 Review.
Cited by
-
Detection of the opening of the bundle crossing in KcsA with fluorescence lifetime spectroscopy reveals the existence of two gates for ion conduction.J Gen Physiol. 2006 Nov;128(5):569-81. doi: 10.1085/jgp.200609638. Epub 2006 Oct 16. J Gen Physiol. 2006. PMID: 17043150 Free PMC article.
-
Concerted gating mechanism underlying KATP channel inhibition by ATP.Biophys J. 2004 Apr;86(4):2101-12. doi: 10.1016/S0006-3495(04)74269-1. Biophys J. 2004. PMID: 15041650 Free PMC article.
-
Fine-tuning of voltage sensitivity of the Kv1.2 potassium channel by interhelix loop dynamics.J Biol Chem. 2013 Apr 5;288(14):9686-9695. doi: 10.1074/jbc.M112.437483. Epub 2013 Feb 14. J Biol Chem. 2013. PMID: 23413033 Free PMC article.
-
The cooperative voltage sensor motion that gates a potassium channel.J Gen Physiol. 2005 Jan;125(1):57-69. doi: 10.1085/jgp.200409197. J Gen Physiol. 2005. PMID: 15623895 Free PMC article.
-
Opening of capsaicin receptor TRPV1 is stabilized equally by its four subunits.J Biol Chem. 2023 Jun;299(6):104828. doi: 10.1016/j.jbc.2023.104828. Epub 2023 May 15. J Biol Chem. 2023. PMID: 37196769 Free PMC article.
References
-
- Ayer RK, Sigworth FJ. Enhanced closed-state inactivation in a mutant Shaker K+channel. J Membr Biol. 1997;157:215–230. - PubMed

