Ligand-insensitive state of cardiac ATP-sensitive K+ channels. Basis for channel opening
- PMID: 9450949
- PMCID: PMC2222775
- DOI: 10.1085/jgp.111.2.381
Ligand-insensitive state of cardiac ATP-sensitive K+ channels. Basis for channel opening
Abstract
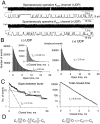
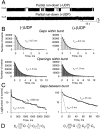
The mechanism by which ATP-sensitive K+ (KATP) channels open in the presence of inhibitory concentrations of ATP remains unknown. Herein, using a four-state kinetic model, we found that the nucleotide diphosphate UDP directed cardiac KATP channels to operate within intraburst transitions. These transitions are not targeted by ATP, nor the structurally unrelated sulfonylurea glyburide, which inhibit channel opening by acting on interburst transitions. Therefore, the channel remained insensitive to ATP and glyburide in the presence of UDP. "Rundown" of channel activity decreased the efficacy with which UDP could direct and maintain the channel to operate within intraburst transitions. Under this condition, the channel was sensitive to inhibition by ATP and glyburide despite the presence of UDP. This behavior of the KATP channel could be accounted for by an allosteric model of ligand-channel interaction. Thus, the response of cardiac KATP channels towards inhibitory ligands is determined by the relative lifetime the channel spends in a ligand-sensitive versus -insensitive state. Interconversion between these two conformational states represents a novel basis for KATP channel opening in the presence of inhibitory concentrations of ATP in a cardiac cell.
Figures
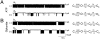

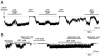

Similar articles
-
Operative condition-dependent response of cardiac ATP-sensitive K+ channels toward sulfonylureas.Circ Res. 1998 Feb 9;82(2):272-8. doi: 10.1161/01.res.82.2.272. Circ Res. 1998. PMID: 9468198
-
Cytoplasmic terminus domains of Kir6.x confer different nucleotide-dependent gating on the ATP-sensitive K+ channel.J Physiol. 1998 Oct 15;512 ( Pt 2)(Pt 2):395-406. doi: 10.1111/j.1469-7793.1998.395be.x. J Physiol. 1998. PMID: 9763630 Free PMC article.
-
A disrupter of actin microfilaments impairs sulfonylurea-inhibitory gating of cardiac KATP channels.Am J Physiol. 1996 Dec;271(6 Pt 2):H2710-6. doi: 10.1152/ajpheart.1996.271.6.H2710. Am J Physiol. 1996. PMID: 8997334
-
Pathophysiological functions of ATP-sensitive K+ channels in myocardial ischemia.Jpn Heart J. 1997 May;38(3):297-315. doi: 10.1536/ihj.38.297. Jpn Heart J. 1997. PMID: 9290566 Review.
-
Cardiac ATP-sensitive K+ channels: regulation by intracellular nucleotides and K+ channel-opening drugs.Am J Physiol. 1995 Sep;269(3 Pt 1):C525-45. doi: 10.1152/ajpcell.1995.269.3.C525. Am J Physiol. 1995. PMID: 7573382 Review.
Cited by
-
Modulation of K(ATP) currents in rat ventricular myocytes by hypoxia and a redox reaction.Acta Pharmacol Sin. 2009 Oct;30(10):1399-414. doi: 10.1038/aps.2009.134. Acta Pharmacol Sin. 2009. PMID: 19801996 Free PMC article.
-
Sulfonylureas suppress the stimulatory action of Mg-nucleotides on Kir6.2/SUR1 but not Kir6.2/SUR2A KATP channels: a mechanistic study.J Gen Physiol. 2014 Nov;144(5):469-86. doi: 10.1085/jgp.201411222. J Gen Physiol. 2014. PMID: 25348414 Free PMC article.
-
Ligand-dependent linkage of the ATP site to inhibition gate closure in the KATP channel.J Gen Physiol. 2005 Sep;126(3):285-99. doi: 10.1085/jgp.200509289. J Gen Physiol. 2005. PMID: 16129775 Free PMC article.
-
Phosphoinositides decrease ATP sensitivity of the cardiac ATP-sensitive K(+) channel. A molecular probe for the mechanism of ATP-sensitive inhibition.J Gen Physiol. 1999 Aug;114(2):251-69. doi: 10.1085/jgp.114.2.251. J Gen Physiol. 1999. PMID: 10436001 Free PMC article.
-
Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels.Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7623-8. doi: 10.1073/pnas.121038198. Epub 2001 Jun 5. Proc Natl Acad Sci U S A. 2001. PMID: 11390963 Free PMC article.
References
-
- Aguilar-Bryan L, Nichols CG, Rajan AS, Parker C, Bryan J. Co-expression of sulfonylurea receptors and KATPchannels in hamster insulinoma tumor (HIT) cells. Evidence for direct association of the receptor with the channel. J Biol Chem. 1992;267:14934–14940. - PubMed
-
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, IV, Boyd AE, III, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. - PubMed
-
- Alekseev AE, Gomez LA, Aleksandrova LA, Brady PA, Terzic A. Opening of cardiac sarcolemmal KATPchannels by dinitrophenol separate from metabolic inhibition. J Membr Biol. 1997a;157:203–214. - PubMed
-
- Alekseev AE, Kennedy ME, Navarro B, Terzic A. Burst kinetics of co-expressed Kir6.2/SUR1 clones: comparison of recombinant with native ATP-sensitive K+channel behavior. J Membr Biol. 1997b;159:161–168. - PubMed
-
- Alekseev AE, Jovanovic A, Lopez JR, Terzic A. Adenosine slows the rate of K+-induced membrane depolarization in ventricular cardiomyocytes: possible implication in hyperkalemic cardioplegia. J Mol Cell Cardiol. 1996a;28:1193–1202. - PubMed

