14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts
- PMID: 9450960
- PMCID: PMC25261
- DOI: 10.1091/mbc.9.2.345
14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts
Abstract
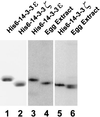
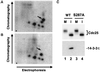
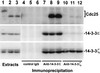
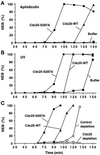
Cdc25, the dual-specificity phosphatase that dephosphorylates the Cdc2-cyclin B complex at mitosis, is highly regulated during the cell cycle. In Xenopus egg extracts, Cdc25 is associated with two isoforms of the 14-3-3 protein. Cdc25 is complexed primarily with 14-3-3epsilon and to a lesser extent with 14-3-3zeta. The association of these 14-3-3 proteins with Cdc25 varies dramatically during the cell cycle: binding is high during interphase but virtually absent at mitosis. Interaction with 14-3-3 is mediated by phosphorylation of Xenopus Cdc25 at Ser-287, which resides in a consensus 14-3-3 binding site. Recombinant Cdc25 with a point mutation at this residue (Cdc25-S287A) is incapable of binding to 14-3-3. Addition of the Cdc25-S287A mutant to Xenopus egg extracts accelerates mitosis and overrides checkpoint-mediated arrests of mitotic entry due to the presence of unreplicated and damaged DNA. These findings indicate that 14-3-3 proteins act as negative regulators of Cdc25 in controlling the G2-M transition.
Figures
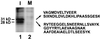


References
-
- Aitken A. 14-3-3 and its possible role in co-ordinating multiple signalling pathways. Trends Cell Biol. 1996;6:341–347. - PubMed
-
- Boyle WJ, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. - PubMed
-
- Carpenter PB, Mueller PR, Dunphy WG. Role for a Xenopus Orc2-related protein in controlling DNA replication. Nature. 1996;379:357–360. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

