Wortmannin-sensitive phosphorylation, translocation, and activation of PLCgamma1, but not PLCgamma2, in antigen-stimulated RBL-2H3 mast cells
- PMID: 9450969
- PMCID: PMC25278
- DOI: 10.1091/mbc.9.2.483
Wortmannin-sensitive phosphorylation, translocation, and activation of PLCgamma1, but not PLCgamma2, in antigen-stimulated RBL-2H3 mast cells
Abstract
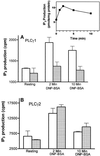
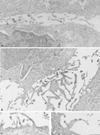
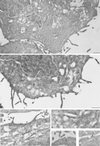
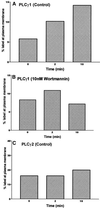
In RBL-2H3 tumor mast cells, cross-linking the high affinity IgE receptor (FcepsilonRI) with antigen activates cytosolic tyrosine kinases and stimulates Ins(1,4,5)P3 production. Using immune complex phospholipase assays, we show that FcepsilonRI cross-linking activates both PLCgamma1 and PLCgamma2. Activation is accompanied by the increased phosphorylation of both PLCgamma isoforms on serine and tyrosine in antigen-treated cells. We also show that the two PLCgamma isoforms have distinct subcellular localizations. PLCgamma1 is primarily cytosolic in resting RBL-2H3 cells, with low levels of plasma membrane association. After antigen stimulation, PLCgamma1 translocates to the plasma membrane where it associates preferentially with membrane ruffles. In contrast, PLCgamma2 is concentrated in a perinuclear region near the Golgi and adjacent to the plasma membrane in resting cells and does not redistribute appreciably after FcepsilonRI cross-linking. The activation of PLCgamma1, but not of PLCgamma2, is blocked by wortmannin, a PI 3-kinase inhibitor previously shown to block antigen-stimulated ruffling and to inhibit Ins(1,4,5)P3 synthesis. In addition, wortmannin strongly inhibits the antigen-stimulated phosphorylation of both serine and tyrosine residues on PLCgamma1 with little inhibition of PLCgamma2 phosphorylation. Wortmannin also blocks the antigen-stimulated translocation of PLCgamma1 to the plasma membrane. Our results implicate PI 3-kinase in the phosphorylation, translocation, and activation of PLCgamma1. Although less abundant than PLCgamma2, activated PLCgamma1 may be responsible for the bulk of antigen-stimulated Ins(1,4,5)P3 production in RBL-2H3 cells.
Figures




References
-
- Atkinson TP, Kaliner MA, Hohman RJ. Phospholipase Cγ1 is translocated to the membrane of rat basophilic leukemia cells in response to aggregation of IgE receptors. J Immunol. 1992;148:2194–2200. - PubMed
-
- Atkinson TP, Lee C-W, Rhee SG, Hohman RJ. Orthovanadate induces translocation of phospholipase C-γ1 and -γ2 in permeabilized mast cells. J Immunol. 1993;151:1448–1455. - PubMed
-
- Bar-Sagi D, Rotin D, Batzer A, Mandiya V, Schlessinger J. SH3 domains direct cellular localization of signaling molecules. Cell. 1993;74:83–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

