Proteolytic processing and Ca2+-binding activity of dense-core vesicle polypeptides in Tetrahymena
- PMID: 9450970
- PMCID: PMC25279
- DOI: 10.1091/mbc.9.2.497
Proteolytic processing and Ca2+-binding activity of dense-core vesicle polypeptides in Tetrahymena
Abstract
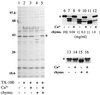
Formation and discharge of dense-core secretory vesicles depend on controlled rearrangement of the core proteins during their assembly and dispersal. The ciliate Tetrahymena thermophila offers a simple system in which the mechanisms may be studied. Here we show that most of the core consists of a set of polypeptides derived proteolytically from five precursors. These share little overall amino acid identity but are nonetheless predicted to have structural similarity. In addition, sites of proteolytic processing are notably conserved and suggest that specific endoproteases as well as carboxypeptidase are involved in core maturation. In vitro binding studies and sequence analysis suggest that the polypeptides bind calcium in vivo. Core assembly and postexocytic dispersal are compartment-specific events. Two likely regulatory factors are proteolytic processing and exposure to calcium. We asked whether these might directly influence the conformations of core proteins. Results using an in vitro chymotrypsin accessibility assay suggest that these factors can induce sequential structural rearrangements. Such progressive changes in polypeptide folding may underlie the mechanisms of assembly and of rapid postexocytic release. The parallels between dense-core vesicles in different systems suggest that similar mechanisms are widespread in this class of organelles.
Figures






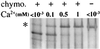




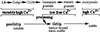
Similar articles
-
Granule lattice protein 1 (Grl1p), an acidic, calcium-binding protein in Tetrahymena thermophila dense-core secretory granules, influences granule size, shape, content organization, and release but not protein sorting or condensation.J Cell Biol. 1996 Dec;135(6 Pt 2):1775-87. doi: 10.1083/jcb.135.6.1775. J Cell Biol. 1996. PMID: 8991090 Free PMC article.
-
Proprotein processing within secretory dense core granules of Tetrahymena thermophila.J Biol Chem. 2003 Feb 7;278(6):4087-95. doi: 10.1074/jbc.M207236200. Epub 2002 Nov 14. J Biol Chem. 2003. PMID: 12435750
-
Genetic, genomic, and functional analysis of the granule lattice proteins in Tetrahymena secretory granules.Mol Biol Cell. 2005 Sep;16(9):4046-60. doi: 10.1091/mbc.e05-01-0028. Epub 2005 Jun 15. Mol Biol Cell. 2005. PMID: 15958493 Free PMC article.
-
Protein processing and morphogenesis of secretory granules in Paramecium.Biochimie. 1994;76(3-4):329-35. doi: 10.1016/0300-9084(94)90167-8. Biochimie. 1994. PMID: 7819344 Review.
-
Out with a bang! Tetrahymena as a model system to study secretory granule biogenesis.Traffic. 2004 Feb;5(2):63-8. doi: 10.1046/j.1600-0854.2003.00155.x. Traffic. 2004. PMID: 14690495 Review.
Cited by
-
Secretion of Polypeptide Crystals from Tetrahymena thermophila Secretory Organelles (Mucocysts) Depends on Processing by a Cysteine Cathepsin, Cth4p.Eukaryot Cell. 2015 Aug;14(8):817-33. doi: 10.1128/EC.00058-15. Epub 2015 Jun 19. Eukaryot Cell. 2015. PMID: 26092918 Free PMC article.
-
New class of cargo protein in Tetrahymena thermophila dense core secretory granules.Eukaryot Cell. 2002 Aug;1(4):583-93. doi: 10.1128/EC.1.4.583-593.2002. Eukaryot Cell. 2002. PMID: 12456006 Free PMC article.
-
An antisense approach to phenotype-based gene cloning in Tetrahymena.Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8709-13. doi: 10.1073/pnas.151243498. Epub 2001 Jul 3. Proc Natl Acad Sci U S A. 2001. PMID: 11438705 Free PMC article.
-
An evolutionary balance: conservation vs innovation in ciliate membrane trafficking.Traffic. 2017 Jan;18(1):18-28. doi: 10.1111/tra.12450. Epub 2016 Oct 27. Traffic. 2017. PMID: 27696651 Free PMC article. Review.
-
An endosomal syntaxin and the AP-3 complex are required for formation and maturation of candidate lysosome-related secretory organelles (mucocysts) in Tetrahymena thermophila.Mol Biol Cell. 2017 Jun 1;28(11):1551-1564. doi: 10.1091/mbc.E17-01-0018. Epub 2017 Apr 5. Mol Biol Cell. 2017. PMID: 28381425 Free PMC article.
References
-
- Adoutte A. Exocytosis: Biogenesis, Transport and Secretion of Trichocysts. In: Görtz HD, editor. Paramecium. Berlin: Springer-Verlag; 1988. pp. 325–362.
-
- Adoutte A, Garreau de Loubresse N, Beisson J. Proteolytic cleavage and maturation of the crystalline secretion products of Paramecium. J Mol Biol, 1984;180:1065–1081. - PubMed
-
- Anderer R, Hausmann K. Properties and structure of isolated extrusive organelles. J Ultrastruct Res, 1977;60:21–26. - PubMed
-
- Arvan P, Castle JD. Protein sorting and secretion granule formation in regulated secretory cells. Trends Cell Biol, 1992;2:327–331. - PubMed
-
- Attanoos RL, Allen AK. The characterization of the proteins which are secreted by the mucocysts of Tetrahymena thermophila. Biochim Biophys Acta, 1987;924:154–158.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

