Proteolytic processing and Ca2+-binding activity of dense-core vesicle polypeptides in Tetrahymena
- PMID: 9450970
- PMCID: PMC25279
- DOI: 10.1091/mbc.9.2.497
Proteolytic processing and Ca2+-binding activity of dense-core vesicle polypeptides in Tetrahymena
Abstract
Formation and discharge of dense-core secretory vesicles depend on controlled rearrangement of the core proteins during their assembly and dispersal. The ciliate Tetrahymena thermophila offers a simple system in which the mechanisms may be studied. Here we show that most of the core consists of a set of polypeptides derived proteolytically from five precursors. These share little overall amino acid identity but are nonetheless predicted to have structural similarity. In addition, sites of proteolytic processing are notably conserved and suggest that specific endoproteases as well as carboxypeptidase are involved in core maturation. In vitro binding studies and sequence analysis suggest that the polypeptides bind calcium in vivo. Core assembly and postexocytic dispersal are compartment-specific events. Two likely regulatory factors are proteolytic processing and exposure to calcium. We asked whether these might directly influence the conformations of core proteins. Results using an in vitro chymotrypsin accessibility assay suggest that these factors can induce sequential structural rearrangements. Such progressive changes in polypeptide folding may underlie the mechanisms of assembly and of rapid postexocytic release. The parallels between dense-core vesicles in different systems suggest that similar mechanisms are widespread in this class of organelles.
Figures






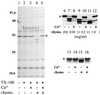
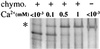




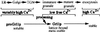
References
-
- Adoutte A. Exocytosis: Biogenesis, Transport and Secretion of Trichocysts. In: Görtz HD, editor. Paramecium. Berlin: Springer-Verlag; 1988. pp. 325–362.
-
- Adoutte A, Garreau de Loubresse N, Beisson J. Proteolytic cleavage and maturation of the crystalline secretion products of Paramecium. J Mol Biol, 1984;180:1065–1081. - PubMed
-
- Anderer R, Hausmann K. Properties and structure of isolated extrusive organelles. J Ultrastruct Res, 1977;60:21–26. - PubMed
-
- Arvan P, Castle JD. Protein sorting and secretion granule formation in regulated secretory cells. Trends Cell Biol, 1992;2:327–331. - PubMed
-
- Attanoos RL, Allen AK. The characterization of the proteins which are secreted by the mucocysts of Tetrahymena thermophila. Biochim Biophys Acta, 1987;924:154–158.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

