Diffusely adhering Escherichia coli strains induce attaching and effacing phenotypes and secrete homologs of Esp proteins
- PMID: 9453606
- PMCID: PMC107938
- DOI: 10.1128/IAI.66.2.528-539.1998
Diffusely adhering Escherichia coli strains induce attaching and effacing phenotypes and secrete homologs of Esp proteins
Abstract
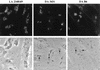
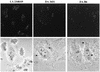
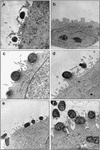
Recent epidemiological studies indicate that Escherichia coli strains which exhibit the diffuse-adherence phenotype (DAEC strains) represent a potential cause of diarrhea in infants. We investigated the interaction of DAEC strains isolated from diarrhea patients in Brazil and in Germany with epithelial cells in tissue culture. The investigated strains were identified as DAEC strains by (i) their attachment pattern, (ii) presence of genes associated with the Dr family of adhesins, and (iii) lack of genetic markers for other diarrhea-associated E. coli categories. Several clinical DAEC isolates were shown to secrete similar patterns of proteins into tissue culture medium. Protein secretion was found to be regulated by environmental parameters, namely, medium, temperature, pH, and iron concentration. DAEC strains secreting these proteins induced accumulation of actin and tyrosine-phosphorylated proteins at sites of bacterial attachment, leading to the formation of pedestals and/or extended surface structures. These changes were phenotypically similar to the attaching and effacing (A/E) lesions observed with enteropathogenic and some enterohemorrhagic E. coli strains carrying the locus of enterocyte effacement (LEE) pathogenicity island. Proteins homologous to the EspA, EspB, and EspD proteins, necessary for signal transduction events inducing A/E lesions, were identified by sequence analysis and cross-reaction of specific antibodies. However, initially nonadhering strains secreting these proteins induced signal transduction events only after prolonged infection. These results indicate that secretion of the Esp proteins alone is not sufficient for efficient signal transduction. This study further shows that some DAEC strains are likely to contain a homolog(s) of the LEE locus which may contribute to the pathogenic potential of DAEC.
Figures





References
-
- Aleksic S, Karch H, Bockemuhl J. A biotyping scheme for Shiga-like (Vero) toxin-producing Escherichia coli O157 and a list of serological cross-reactions between O157 and other gram-negative bacteria. Int J Med Microbiol Virol Parasitol Infect Dis. 1992;276:221–230. - PubMed
-
- Baqui A H, Sack R B, Black R E, Haider K, Hossain A, Alim A R, Yunus M, Chowdhury H R, Siddique A K. Enteropathogens associated with acute and persistent diarrhea in Bangladeshi children less than 5 years of age. J Infect Dis. 1992;166:792–796. - PubMed
-
- Benz I, Schmidt M A. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol Microbiol. 1992;6:1539–1546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

