Mapping of a protective epitope of the CopB outer membrane protein of Moraxella catarrhalis
- PMID: 9453607
- PMCID: PMC107939
- DOI: 10.1128/IAI.66.2.540-548.1998
Mapping of a protective epitope of the CopB outer membrane protein of Moraxella catarrhalis
Abstract
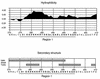
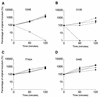
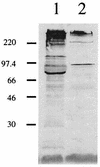
A monoclonal antibody (MAb) (MAb 10F3) directed against the CopB outer membrane protein of Moraxella catarrhalis previously was found to enhance pulmonary clearance of M. catarrhalis in an animal model (M. Helminen, I. Maciver, J. L. Latimer, L. D. Cope, G. H. McCracken, Jr., and E. J. Hansen, Infect. Immun. 61:2003-2010, 1993). In the present study, this same MAb was shown to exert complement-dependent bactericidal activity against this pathogen in vitro. Nucleotide sequence analysis of the copB gene from two MAb 10F3-reactive and two MAb 10F3-unreactive strains of M. catarrhalis revealed that the deduced amino acid sequences of these four CopB proteins were at least 90% identical. Comparison of the amino acid sequences of these proteins allowed localization of possible MAb 10F3 binding sites to five relatively small regions of the CopB protein from M. catarrhalis O35E. When five synthetic peptides representing these regions were tested for their ability to bind MAb 10F3 in a direct enzyme-linked immunosorbent assay system, an oligopeptide containing 26 amino acids was shown to bind this MAb. The actual binding region for MAb 10F3 was localized further through the use of overlapping decapeptides that spanned this 26-mer. A fusion protein containing the same 26-mer readily bound MAb 10F3 and was used to immunize mice. The resultant antiserum contained antibodies that reacted with the CopB protein of the homologous M. catarrhalis strain in Western blot analysis and bound to the surface of both homologous and heterologous strains of M. catarrhalis.
Figures

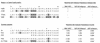


References
-
- Anonymous. Consensus. Pediatr Infect Dis J. 1989;8:S94–S97.
-
- Aebi C, Cope L D, Latimer J L, Thomas S E, Slaughter C A, McCracken G H, Hansen E J. Abstracts of the 36th International Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Moraxella catarrhalis outer membrane protein CopB: identification of a peptide that contains a protective epitope, abstr. 783; p. 158.
-
- Aebi C, Stone B, Beucher M, Cope L D, Maciver I, Thomas S E, McCracken G H, Jr, Sparling P F, Hansen E J. Expression of the CopB outer membrane protein by Moraxella catarrhalis is regulated by iron and affects iron acquisition from transferrin and lactoferrin. Infect Immun. 1996;64:2024–2030. - PMC - PubMed
-
- Aspin M M, Hoberman A, McCarty J, McLinn S E, Aronoff S, Lang D J, Arrieta A. Comparative study of the safety and efficacy of clarithromycin and amoxicillin-clavulanate in the treatment of acute otitis media in children. J Pediatr. 1995;126:677–678. - PubMed
-
- Bartos L C, Murphy T F. Comparison of the outer membrane proteins of 50 strains of Branhamella catarrhalis. J Infect Dis. 1988;158:761–765. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

