Coiling phagocytosis discriminates between different spirochetes and is enhanced by phorbol myristate acetate and granulocyte-macrophage colony-stimulating factor
- PMID: 9453619
- PMCID: PMC107950
- DOI: 10.1128/IAI.66.2.627-635.1998
Coiling phagocytosis discriminates between different spirochetes and is enhanced by phorbol myristate acetate and granulocyte-macrophage colony-stimulating factor
Abstract
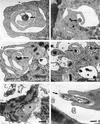
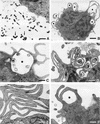
The mechanisms involved in coiling phagocytosis are not yet known, and it is not even clear whether this phenomenon is either an incidental event or a specific response. Therefore, the phagocytic uptake of Borrelia burgdorferi and other spirochetes by human monocytes in vitro was used to investigate the involvement of both sides--microbes and phagocytes--in coiling phagocytosis. As seen with electron microscopy, morphologically similar Borrelia, Leptospira and Treponema strains induced markedly different frequencies of coiling phagocytosis. The monocytes used coiling phagocytosis for both live (motile) and killed (nonmotile) B. burgdorferi, but pseudopod coils were observed neither with fragmented B. burgdorferi nor with cell-free supernatant from B. burgdorferi cultures. Investigation of the relationship of coiling phagocytosis with other pseudopod-based cellular mechanisms revealed that the use of bioreagents that inhibit conventional phagocytosis also inhibited coiling phagocytis but did not affect membrane ruffling. Bioreagents that increase membrane ruffling did not affect phagocytosis of B. burgdorferi, except for granulocyte-macrophage colony-stimulating factor and phorbol myristate acetate, which increased coiling phagocytosis selectively. These results demonstrate that coiling phagocytosis is not induced by microbial motility, viability, or a certain morphology and that it is not a random event. Rather, it is a selective uptake mechanism actively driven by the phagocytes. However, whether coiling phagocytosis represents an independent alternative to conventional phagocytosis or, alternatively, a fault in conventional phagocytosis remains to be determined.
Figures





Similar articles
-
Molecular Mechanisms of Borrelia burgdorferi Phagocytosis and Intracellular Processing by Human Macrophages.Biology (Basel). 2021 Jun 22;10(7):567. doi: 10.3390/biology10070567. Biology (Basel). 2021. PMID: 34206480 Free PMC article. Review.
-
Coiling phagocytosis is the preferential phagocytic mechanism for Borrelia burgdorferi.Infect Immun. 1992 Oct;60(10):4205-12. doi: 10.1128/iai.60.10.4205-4212.1992. Infect Immun. 1992. PMID: 1398932 Free PMC article.
-
Phagocytes from both vertebrate and invertebrate species use "coiling" phagocytosis.Dev Comp Immunol. 1996 Nov-Dec;20(6):393-406. doi: 10.1016/s0145-305x(96)00023-7. Dev Comp Immunol. 1996. PMID: 9040982
-
Coiling phagocytosis of Borrelia burgdorferi by primary human macrophages is controlled by CDC42Hs and Rac1 and involves recruitment of Wiskott-Aldrich syndrome protein and Arp2/3 complex.Infect Immun. 2001 Mar;69(3):1739-46. doi: 10.1128/IAI.69.3.1739-1746.2001. Infect Immun. 2001. PMID: 11179351 Free PMC article.
-
Actin-Dependent Regulation of Borrelia burgdorferi Phagocytosis by Macrophages.Curr Top Microbiol Immunol. 2017;399:133-154. doi: 10.1007/82_2016_26. Curr Top Microbiol Immunol. 2017. PMID: 27744511 Review.
Cited by
-
Molecular Mechanisms of Borrelia burgdorferi Phagocytosis and Intracellular Processing by Human Macrophages.Biology (Basel). 2021 Jun 22;10(7):567. doi: 10.3390/biology10070567. Biology (Basel). 2021. PMID: 34206480 Free PMC article. Review.
-
Phagosomal TLR signaling upon Borrelia burgdorferi infection.Front Cell Infect Microbiol. 2014 May 20;4:55. doi: 10.3389/fcimb.2014.00055. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24904837 Free PMC article. Review.
-
The formins FMNL1 and mDia1 regulate coiling phagocytosis of Borrelia burgdorferi by primary human macrophages.Infect Immun. 2013 May;81(5):1683-95. doi: 10.1128/IAI.01411-12. Epub 2013 Mar 4. Infect Immun. 2013. PMID: 23460512 Free PMC article.
-
Legionella pneumophila entry gene rtxA is involved in virulence.Infect Immun. 2001 Jan;69(1):508-17. doi: 10.1128/IAI.69.1.508-517.2001. Infect Immun. 2001. PMID: 11119544 Free PMC article.
-
Coiling phagocytosis of trypanosomatids and fungal cells.Infect Immun. 1998 Sep;66(9):4331-9. doi: 10.1128/IAI.66.9.4331-4339.1998. Infect Immun. 1998. PMID: 9712785 Free PMC article.
References
-
- Allen L A H, Aderem A. Mechanisms of phagocytosis. Curr Opin Immunol. 1996;8:36–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

