Treponema denticola outer membrane inhibits calcium flux in gingival fibroblasts
- PMID: 9453630
- PMCID: PMC107960
- DOI: 10.1128/IAI.66.2.703-709.1998
Treponema denticola outer membrane inhibits calcium flux in gingival fibroblasts
Abstract
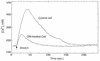
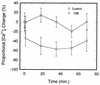
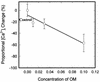
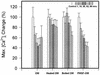
Treponema denticola is a cultivable oral spirochete which perturbs the cytoskeleton in cultured cells of oral origin, but intracellular signalling pathways by which it affects actin assembly are largely unknown. As the outer membrane (OM) of Treponema denticola disrupts actin-dependent processes that normally require precise control of intracellular calcium, we studied the effects of an OM extract on internal calcium release, ligand-gated and calcium release-activated calcium channels, and related mechanosensitive cation fluxes in human gingival fibroblasts (HGF). Single-cell ratio fluorimetry demonstrated that in resting cells loaded with Fura-2, baseline intracellular Ca2+ concentration ([Ca2+]i) was not affected by treatment with OM extract, but normal spontaneous [Ca2+]i oscillations were dramatically increased in frequency for 20 to 30 min followed by complete blockade. OM extract inhibited ATP-induced and thapsigargin-induced release of calcium from intracellular stores by 40 and 30%, respectively. Addition of Ca2+ to the extracellular pool following depletion of intracellular Ca2+ by thapsigargin and extracellular Ca2+ by EGTA yielded 59% less replenishment of [Ca2+]i in OM extract-treated than in control HGF. In cells loaded with collagen-coated ferric oxide beads to stimulate integrin-dependent calcium release, baseline [Ca2+]i was nearly doubled but was not significantly different in control and OM extract-treated cells. Magnetically generated tensile forces on the beads induced >300% increases of [Ca2+]i above baseline. Cells preincubated with OM extract exhibited dose-dependent and time-dependent reductions in stretch-induced [Ca2+]i transients, which were due to neither loss of beads from the cells nor cell death. The T. denticola OM inhibitory activity was eliminated by heating the OM extract to 60 degrees C and by boiling but not by phenylmethylsulfonyl fluoride treatment. Thus nonlipopolysaccharide, nonchymotrypsin, heat-sensitive protein(s) in T. denticola OM can evidently inhibit both release of calcium from internal stores and uptake of calcium through the plasma membrane, possibly by interference with calcium release-activated channels.
Figures




References
-
- Arora P D, Bibby K J, McCulloch C A G. Slow oscillations of free intracellular calcium ion concentration in human fibroblasts responding to mechanical stretch. J Cell Physiol. 1994;161:187–200. - PubMed
-
- Arora P D, McCulloch C A G. Dependence of fibroblast migration on actin severing activity of gelsolin. J Biol Chem. 1996;271:20516–20523. - PubMed
-
- Berridge M J. Calcium oscillations. J Biol Chem. 1990;265:9583–9586. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

