Comprehensive study of the intestinal stage of listeriosis in a rat ligated ileal loop system
- PMID: 9453636
- PMCID: PMC107965
- DOI: 10.1128/IAI.66.2.747-755.1998
Comprehensive study of the intestinal stage of listeriosis in a rat ligated ileal loop system
Abstract
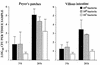
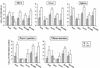
The intestinal stage of listeriosis was studied in a rat ligated ileal loop system. Listeria monocytogenes translocated to deep organs with similar efficiencies after inoculation of loops with or without Peyer's patches. Bacterial seeding of deep organs was demonstrated as early as 15 min after inoculation. It was dose dependent and nonspecific, as the delta inlAB, the delta hly, and the delta actA L. monocytogenes mutants and the nonpathogenic species, Listeria innocua, translocated similarly to wild-type L. monocytogenes strains. The levels of uptake of listeriae by Peyer's patches and villous intestine were similar and low, 50 to 250 CFU per cm2 of tissue. No listeria cells crossing the epithelial sheet of Peyer's patches and villous intestine were observed by transmission electron microscopy. The lack of significant interaction of listeriae and the follicle-associated epithelium of Peyer's patches was confirmed by scanning electron microscopy. The follicular tissue of Peyer's patches was a preferential site of Listeria replication. With all doses tested, the rate of bacterial growth was 10 to 20 times higher in Peyer's patches than in villous intestine. At early stages of Peyer's patch infection, listeriae were observed inside mononuclear cells of the dome area. Listeriae then disseminated throughout the follicular tissue except for the germinal center. The virulence determinants hly and, to a lesser extent, actA, but not inlAB, were required for the completion of this process. This study suggests that Peyer's patches are preferential sites for replication rather than for entry of L. monocytogenes, due to the presence of highly permissive mononuclear cells whose nature remains to be defined.
Figures






References
-
- Amerongen H, Weltzin R, Farnet C, Michetti P, Haseltine W, Neutra M. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J Acquired Immune Defic Syndr. 1991;4:760–765. - PubMed
-
- Barza M. Listeriosis and milk. N Engl J Med. 1985;312:438–440. - PubMed
-
- Berg R D. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995;3:149–154. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

