Increased resistance to Taenia crassiceps murine cysticercosis in Qa-2 transgenic mice
- PMID: 9453638
- PMCID: PMC107967
- DOI: 10.1128/IAI.66.2.760-764.1998
Increased resistance to Taenia crassiceps murine cysticercosis in Qa-2 transgenic mice
Abstract
We previously reported important differences in resistance to Taenia crassiceps murine cysticercosis between BALB/c substrains. It was suggested that resistance might correlate with expression of the nonclassic class I major histocompatibility complex (MHC) Qa-2 antigen; BALB/cAnN is Qa-2 negative and highly susceptible to T. crassiceps, whereas BALB/cJ expresses Qa-2 and is highly resistant. In this study, we investigated the role of Qa-2 in mediating resistance to cysticercosis by linkage analysis and infection of Qa-2 transgenic mice. In BALB/cAnN x (C57BL/6J x BALB/cAnN)F1 and BALB/cAnN x (BALB/cJ x BALB/cAnN)F1 backcrosses, the expression of Qa-2 antigen correlated with resistance to cysticercosis. Significantly fewer parasites were recovered from infected Qa-2 transgenic male and female mice than from nontransgenic mice of similar genetic background. These results clearly demonstrate that the Qa-2 MHC antigen is involved in resistance to T. crassiceps cysticercosis.
Figures
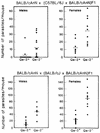

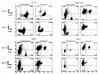
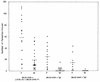
References
-
- Aluja A. Frequency of porcine cysticercosis in Mexico. In: Flisser A, Willms K, Laclette J P, Larralde C, Ridaura C, Beltrán F, editors. Cysticercosis: present state of knowledge and perspectives. New York, N.Y: Academic Press; 1982. pp. 53–62.
-
- Fragoso G, Lamoyi E, Mellor A, Lomelí C, Govezensky T, Sciutto E. Genetic control of susceptibility to Taenia crassiceps cysticercosis. Parasitology. 1996;112:119–124. - PubMed
-
- Freeman R S. Studies of the biology of Taenia crassiceps (Zeder, 1800) Rudolphi, 1810 (Cestoda) Can J Zool. 1962;40:969–990.
-
- Hogan B, Costantini F, Lacy E. Manipulating the mouse embryo. A laboratory manual. Cold Spring Harbor Laboratory, N.Y: Cold Spring Harbor Laboratory; 1986.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

