Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current
- PMID: 9454830
- PMCID: PMC6792730
- DOI: 10.1523/JNEUROSCI.18-04-01196.1998
Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current
Abstract
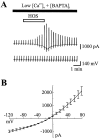
Astrocytes swell during neuronal activity as they accumulate K+ to buffer the increase in external K+ released from neurons. This swelling activates volume-sensitive Cl- channels, which are thought to be important in regulatory volume decrease and in the response of the CNS to trauma and excitotoxicity. Mitogen-activated protein (MAP) kinases also are activated by cell volume changes, but their roles in volume regulation are unknown. We have investigated the role of tyrosine and MAP kinases in the activation of volume-activated Cl- channels in cultured astrocytes, using whole-cell patch-clamp recording and Western immunoblots. As previously described, hypo-osmotic solution induced an outwardly rectifying Cl- current, which was blocked by NPPB and SITS. This Cl- current did not depend on [Ca2+ ]i because it was still observed when 20 mM BAPTA was included in the pipette, but it did exhibit rundown when ATP was omitted. Inhibition of tyrosine kinases with genistein or tyrphostin A23 (but not the inactive agents daidzein and tyrphostin A1) blocked the Cl- current. The MAP kinase kinase (MEK) inhibitor PD 98059 reversibly inhibited activation of the Cl- current by hypo-osmotic solution. Western immunoblots showed that genistein or PD 98059 blocked activation of Erk-1 and Erk-2 by hypo-osmotic solution in astrocytes. Therefore, activation of tyrosine and MAP kinases by swelling is a critical step in the opening of volume-sensitive Cl- channels.
Figures






References
-
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Bakhramov A, Fenech C, Bolton TB. Chloride current activated by hypotonicity in cultured human astrocytoma cells. Exp Physiol. 1995;80:373–389. - PubMed
-
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991;121:101–117. - PubMed
-
- Campbell JS, Seger R, Graves JD, Graves LM, Jensen AM, Krebs EG. The MAP kinase cascade. Recent Prog Horm Res. 1995;50:131–159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
