Synergistic effects of schwann- and muscle-derived factors on motoneuron survival involve GDNF and cardiotrophin-1 (CT-1)
- PMID: 9454853
- PMCID: PMC6792716
- DOI: 10.1523/JNEUROSCI.18-04-01440.1998
Synergistic effects of schwann- and muscle-derived factors on motoneuron survival involve GDNF and cardiotrophin-1 (CT-1)
Abstract
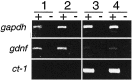
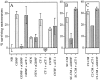
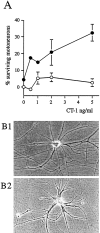
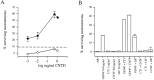
The survival of central neurons depends on multiple neurotrophic factors produced by different cell types. We demonstrate that media conditioned by muscle and Schwann cell lines show strong synergistic effects on survival of purified embryonic day 14.5 rat motoneurons in culture. Different lines of evidence implicate glial cell line-derived neurotrophic factor (GDNF) and cardiotrophin-1 (CT-1) in this synergy. Their expression in the environment of the motoneuron is compartmentalized: gdnf transcripts are expressed principally in Schwann cell lines, whereas ct-1 mRNA is present in myotubes. Blocking antibodies to GDNF inhibit the trophic activity of Schwann cell line-conditioned media by 75%, whereas CT-1 antibodies diminish the myotube-derived activity by 46%. CT-1 and GDNF act synergistically to enhance motoneuron survival in vitro. In vivo, individual motoneurons coexpress both GDNF and CT-1 receptor components. GDNF and CT-1, therefore, are major components of the trophic support provided by the Schwann and muscle cells, respectively. The possibility that they act together on individual motoneurons suggests that the motoneuron must integrate distinct signals from different cellular partners when deciding whether to die or to survive.
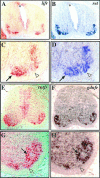
Figures


References
-
- Boutry JM, Hauw JJ, Gansmuller A, Di BN, Pouchelet M, Baron-Van Evercooren A. Establishment and characterization of a mouse Schwann cell line which produces myelin in vivo. J Neurosci Res. 1992;32:15–26. - PubMed
-
- Chandler CE, Parsons LM, Hosang M, Shooter EM. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J Biol Chem. 1984;259:6882–6889. - PubMed
-
- Davis S, Aldrich TH, Valenzuela DM, Wong VV, Furth ME, Squinto SP, Yancopoulos GD. The receptor for ciliary neurotrophic factor. Science. 1991;253:59–63. - PubMed
-
- DeChiara TM, Vejsada R, Poueymirou WT, Acheson A, Suri C, Conover JC, Friedman B, McClain J, Pan L, Stahl N, Ip NY, Kato A, Yancopoulos GD. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell. 1995;83:313–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
