A novel approach for detecting an immunodominant antigen of Porphyromonas gingivalis in diagnosis of adult periodontitis
- PMID: 9455872
- PMCID: PMC121383
- DOI: 10.1128/CDLI.5.1.11-17.1998
A novel approach for detecting an immunodominant antigen of Porphyromonas gingivalis in diagnosis of adult periodontitis
Abstract
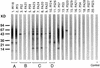
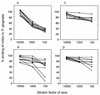
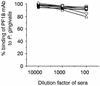
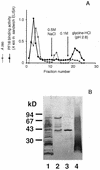
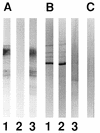
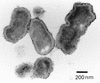
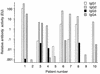
In the course of long-term infection with Porphyromonas gingivalis in adult periodontitis, a specific antibody response to this organism is generated. We describe a potential novel approach for identifying an immunodominant antigen in human periodontitis patients. First, various monoclonal antibodies (MAbs) were established from mice immunized with crude antigen preparations of P. gingivalis FDC 381. The antigen specificities of these MAbs were compared with those of serum antibodies of 10 periodontitis patients in a competitive enzyme-linked immunosorbent assay. The binding of one MAb (termed PF18) was readily inhibited by sera from all patients but not by sera from healthy volunteers. The antigen recognized by PF18 existed on the cell surface, presumably in the capsule layer, shown by immunoelectron microscopic analysis. Purification of the antigenic substance, termed PF18-Ag, was performed by immunoaffinity chromatography with the MAb. Characterization of PF18-Ag suggested that the epitope was composed of carbohydrates but not peptides and that the substance was different from lipopolysaccharide. Measurement of levels of serum antibody to PF18-Ag better discriminated periodontitis patients from healthy individuals than measurement of antibodies to crude antigen preparations of P. gingivalis. Immunoglobulin G2 was the predominant isotype among the antibodies to PF18-Ag in the patients' sera. These results suggest that PF18-Ag, which is possibly a novel substance, is an important antigenic substance and is potentially useful for the clinical diagnosis of adult periodontitis. The approach that was used would also be relevant to detecting immunodominant antigens of other infectious microorganisms.
Figures

Similar articles
-
Preliminary characterisation of antigens recognised by monoclonal antibodies raised to Porphyromonas gingivalis and by sera from patients with periodontitis.J Periodontal Res. 1994 Sep;29(5):339-47. doi: 10.1111/j.1600-0765.1994.tb01232.x. J Periodontal Res. 1994. PMID: 7528274
-
Immunodominant antigens of Porphyromonas gingivalis in patients with rapidly progressive periodontitis.Oral Microbiol Immunol. 1995 Aug;10(4):193-201. doi: 10.1111/j.1399-302x.1995.tb00142.x. Oral Microbiol Immunol. 1995. PMID: 8602330
-
Characterization of the immunodominant antigens of Porphyromonas gingivalis 381 in high-responder patients.Oral Microbiol Immunol. 1996 Aug;11(4):236-41. doi: 10.1111/j.1399-302x.1996.tb00175.x. Oral Microbiol Immunol. 1996. PMID: 9002875
-
Characterization of serum antibodies to Porphyromonas gingivalis in individuals with and without periodontitis.Oral Microbiol Immunol. 1998 Apr;13(2):65-72. doi: 10.1111/j.1399-302x.1998.tb00715.x. Oral Microbiol Immunol. 1998. PMID: 9573796
-
Antibody responses of Porphyromonas gingivalis infected gingivitis and periodontitis subjects.Oral Dis. 1995 Jun;1(2):63-9. doi: 10.1111/j.1601-0825.1995.tb00161.x. Oral Dis. 1995. PMID: 7553387
Cited by
-
Cloning of the gene encoding the Actinobacillus actinomycetemcomitans serotype b OmpA-like outer membrane protein.Infect Immun. 1999 Feb;67(2):942-5. doi: 10.1128/IAI.67.2.942-945.1999. Infect Immun. 1999. PMID: 9916112 Free PMC article.
-
Macrophage Migration Inhibitory Factor (MIF) Supports Homing of Osteoclast Precursors to Peripheral Osteolytic Lesions.J Bone Miner Res. 2016 Sep;31(9):1688-700. doi: 10.1002/jbmr.2854. Epub 2016 Jun 2. J Bone Miner Res. 2016. PMID: 27082509 Free PMC article.
-
Implant Dentistry: Monitoring of Bacteria Along the Transmucosal Passage of the Healing Screw in Absence of Functional Load.Oral Implantol (Rome). 2017 Feb 14;9(Suppl 1/2016 to N 4/2016):10-20. doi: 10.11138/orl/2016.9.1S.010. eCollection 2016 Jan-Mar. Oral Implantol (Rome). 2017. PMID: 28280528 Free PMC article.
-
DC-STAMP Is an Osteoclast Fusogen Engaged in Periodontal Bone Resorption.J Dent Res. 2017 Jun;96(6):685-693. doi: 10.1177/0022034517690490. Epub 2017 Feb 15. J Dent Res. 2017. PMID: 28199142 Free PMC article.
-
B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease.Am J Pathol. 2006 Sep;169(3):987-98. doi: 10.2353/ajpath.2006.060180. Am J Pathol. 2006. PMID: 16936272 Free PMC article.
References
-
- Ban T, Imai K, Yachi A. Immunohistological and immunochemical characterization of a novel pancreatic cancer-associated antigen MUSE11. Cancer Res. 1989;49:7141–7146. - PubMed
-
- Bedi G S, Williams T. Purification and characterization of a collagen-degrading protease from Porphyromonas gingivalis. J Biol Chem. 1994;269:599–606. - PubMed
-
- Blake M S, Johnston K H, Russell J G, Gotschlich E C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984;136:175–179. - PubMed
-
- Cutler C W, Arnold R R, Schenkein H A. Inhibition of C3 and IgG proteolysis enhances phagocytosis of Porphyromonas gingivalis. J Immunol. 1993;151:7016–7029. - PubMed
-
- Cutler C W, Kalmar J R, Genco C A. Pathogenic strategies of oral anaerobe Porphyromonas gingivalis. Trends Microbiol. 1995;3:45–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

