Comparison of neutralizing and hemagglutination-inhibiting antibody responses to influenza A virus vaccination of human immunodeficiency virus-infected individuals
- PMID: 9455891
- PMCID: PMC121402
- DOI: 10.1128/CDLI.5.1.114-117.1998
Comparison of neutralizing and hemagglutination-inhibiting antibody responses to influenza A virus vaccination of human immunodeficiency virus-infected individuals
Abstract
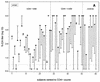
A neutralization enzyme immunoassay (N-EIA) was used to determine the neutralizing serum antibody titers to influenza A/Taiwan/1/86 (H1N1) and Beijing/353/89 (H3N2) viruses after vaccination of 51 human immunodeficiency virus (HIV) type 1-infected individuals and 10 healthy noninfected controls against influenza virus infection. Overall, the N-EIA titers correlated well with the hemagglutination-inhibition (HAI) titers that were observed in the same samples in a previous study (F. P. Kroon, J. T. van Dissel, J. C. de Jong, and R. van Furth, AIDS 8:469-476,1994). The N-EIA appeared to be more sensitive than the HAI test. Significantly more fourfold or higher rises in N-EIA titer and higher mean N-EIA titers occurred in HIV-infected individuals with > or =200 CD4+ cells per microl than in those with <200 CD4+ cells per microl.
Figures



References
-
- Ada G L, Jones P D. The immune response to influenza virus infection. Curr Top Microbiol Immunol. 1986;128:1–54. - PubMed
-
- Arden N H, Cox N J, Schonberger L B. Prevention and control of influenza. Recommendations of the advisory committee on immunization practices (ACIP) Morbid Mortal Weekly Rep. 1997;46:6–7. - PubMed
-
- Centers for Disease Control and Prevention. Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbid Mortal Weekly Rep. 1993;41:1–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

