A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum
- PMID: 9456314
- PMCID: PMC2140172
- DOI: 10.1083/jcb.140.3.525
A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum
Abstract
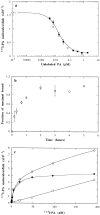
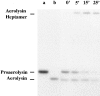
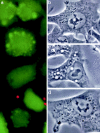
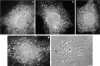
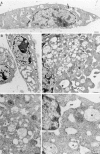
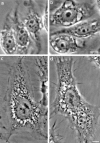
In this paper, we have investigated the effects of the pore-forming toxin aerolysin, produced by Aeromonas hydrophila, on mammalian cells. Our data indicate that the protoxin binds to an 80-kD glycosyl-phosphatidylinositol (GPI)-anchored protein on BHK cells, and that the bound toxin is associated with specialized plasma membrane domains, described as detergent-insoluble microdomains, or cholesterol-glycolipid "rafts." We show that the protoxin is then processed to its mature form by host cell proteases. We propose that the preferential association of the toxin with rafts, through binding to GPI-anchored proteins, is likely to increase the local toxin concentration and thereby promote oligomerization, a step that it is a prerequisite for channel formation. We show that channel formation does not lead to disruption of the plasma membrane but to the selective permeabilization to small ions such as potassium, which causes plasma membrane depolarization. Next we studied the consequences of channel formation on the organization and dynamics of intracellular membranes. Strikingly, we found that the toxin causes dramatic vacuolation of the ER, but does not affect other intracellular compartments. Concomitantly we find that the COPI coat is released from biosynthetic membranes and that biosynthetic transport of newly synthesized transmembrane G protein of vesicular stomatitis virus is inhibited. Our data indicate that binding of proaerolysin to GPI-anchored proteins and processing of the toxin lead to oligomerization and channel formation in the plasma membrane, which in turn causes selective disorganization of early biosynthetic membrane dynamics.
Figures









References
-
- Bergeron JJ, Brenner MB, Thomas DY, Williams DB. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994;19:124–128. - PubMed

