Toxicity of linoleic acid hydroperoxide to Saccharomyces cerevisiae: involvement of a respiration-related process for maximal sensitivity and adaptive response
- PMID: 9457848
- PMCID: PMC106912
- DOI: 10.1128/JB.180.3.483-490.1998
Toxicity of linoleic acid hydroperoxide to Saccharomyces cerevisiae: involvement of a respiration-related process for maximal sensitivity and adaptive response
Abstract
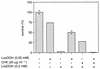
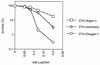
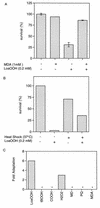
Linoleic acid hydroperoxide (LoaOOH) formed during free radical attack on long-chain unsaturated fatty acids is an important source of biomembrane damage and is implicated in the onset of atherosclerosis, hepatic diseases, and food rancidity. LoaOOH is toxic to wild-type Saccharomyces cerevisiae at a very low concentration (0.2 mM) relative to other peroxides. By using isogenic mutant strains, the possible roles of glutathione (gsh1 and gsh2), glutathione reductase (glr1), respiratory competence ([rho0] petite), and yAP-1p-mediated expression (yap1) in conferring LoaOOH resistance have been examined. Respiration-related processes were essential for maximal toxicity and adaptation, as evidenced by the fact that the [rho0] petite mutant was most resistant to LoaOOH but could not adapt. Furthermore, when respiration was blocked by using inhibitors of respiration and mutants defective in respiratory-chain components, cells became more resistant. An important role for reduced glutathione and yAP-1 in the cellular response to LoaOOH was shown, since the yap1 and glr1 mutants were more sensitive than the wild type. In addition, total glutathione peroxidase activity increased following treatment with LoaOOH, indicating a possible detoxification role for this enzyme. Yeast also showed an adaptive response when pretreated with a nonlethal dose of LoaOOH (0.05 mM) and subsequently treated with a lethal dose (0.2 mM), and de novo protein synthesis was required, since adaptation was abolished upon treatment of cells with cycloheximide (25 microg ml-1). The wild-type adaptive response to LoaOOH was independent of those for the superoxide-generating agents paraquat and menadione and also of those for the organic hydroperoxides cumene hydroperoxide and tert-butyl hydroperoxide. Pretreatment with LoaOOH induced resistance to hydrogen peroxide, while pretreatment of cells with malondialdehyde (a lipid peroxidation product) and heat shock (37 degrees C) gave cross-adaptation to LoaOOH, indicating that yeast has effective overlapping defense systems that can detoxify fatty acid hydroperoxides directly or indirectly.
Figures



Similar articles
-
Proteomic response to linoleic acid hydroperoxide in Saccharomyces cerevisiae.FEMS Yeast Res. 2017 May 1;17(3). doi: 10.1093/femsyr/fox022. FEMS Yeast Res. 2017. PMID: 28449083
-
Saccharomyces cerevisiae exhibits a yAP-1-mediated adaptive response to malondialdehyde.J Bacteriol. 1997 Feb;179(4):1096-101. doi: 10.1128/jb.179.4.1096-1101.1997. J Bacteriol. 1997. PMID: 9023189 Free PMC article.
-
Lipid hydroperoxides activate the mitogen-activated protein kinase Mpk1p in Saccharomyces cerevisiae.J Biol Chem. 2003 Oct 24;278(43):41849-55. doi: 10.1074/jbc.M307760200. Epub 2003 Aug 11. J Biol Chem. 2003. PMID: 12912987
-
Transcriptomic insights into the molecular response of Saccharomyces cerevisiae to linoleic acid hydroperoxide.Free Radic Res. 2013 Dec;47(12):1054-65. doi: 10.3109/10715762.2013.849344. Epub 2013 Oct 21. Free Radic Res. 2013. PMID: 24074273
-
Yeast AP-1 like transcription factors (Yap) and stress response: a current overview.Microb Cell. 2019 May 28;6(6):267-285. doi: 10.15698/mic2019.06.679. Microb Cell. 2019. PMID: 31172012 Free PMC article. Review.
Cited by
-
The genome-wide early temporal response of Saccharomyces cerevisiae to oxidative stress induced by cumene hydroperoxide.PLoS One. 2013 Sep 20;8(9):e74939. doi: 10.1371/journal.pone.0074939. eCollection 2013. PLoS One. 2013. PMID: 24073228 Free PMC article.
-
Mechanism of cytotoxicity of paraquat.Environ Health Prev Med. 2002 Jul;7(3):89-94. doi: 10.1265/ehpm.2002.89. Environ Health Prev Med. 2002. PMID: 21432289 Free PMC article.
-
Transcriptional and antioxidative responses to endogenous polyunsaturated fatty acid accumulation in yeast.Mol Cell Biochem. 2015 Jan;399(1-2):27-37. doi: 10.1007/s11010-014-2229-6. Epub 2014 Oct 4. Mol Cell Biochem. 2015. PMID: 25280400
-
Control of nongenetic heterogeneity in growth rate and stress tolerance of Saccharomyces cerevisiae by cyclic AMP-regulated transcription factors.PLoS Genet. 2018 Nov 2;14(11):e1007744. doi: 10.1371/journal.pgen.1007744. eCollection 2018 Nov. PLoS Genet. 2018. PMID: 30388117 Free PMC article.
-
Differential protein S-thiolation of glyceraldehyde-3-phosphate dehydrogenase isoenzymes influences sensitivity to oxidative stress.Mol Cell Biol. 1999 Apr;19(4):2650-6. doi: 10.1128/MCB.19.4.2650. Mol Cell Biol. 1999. PMID: 10082531 Free PMC article.
References
-
- Babcock G T, Wikstrom M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992;356:301–309. - PubMed
-
- Babizhayev M. Failure to withstand oxidative stress induced by phospholipid hydroperoxides as a possible cause of the lens opacities in systemic diseases and ageing. Biochim Biophys Acta. 1996;1315:87–99. - PubMed
-
- Baker K, Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991;349:205–208. - PubMed
-
- Balzi E, Wang M, Leterme S, Van Dyck L, Goffeau A. PDR5, a novel yeast multidrug resistance conferring transporter controlled by the transcription regulator PDR1. J Biol Chem. 1994;269:2206–2214. - PubMed
-
- Beeor-Tzahar T, Ben-Hayyim G, Holland D, Faltin Z, Eshdat Y. A stress-associated citrus protein is a distinct plant phospholipid hydroperoxide glutathione peroxidase. FEBS Lett. 1995;366:151–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

