Specific in vivo labeling of cell surface-exposed protein loops: reactive cysteines in the predicted gating loop mark a ferrichrome binding site and a ligand-induced conformational change of the Escherichia coli FhuA protein
- PMID: 9457864
- PMCID: PMC106928
- DOI: 10.1128/JB.180.3.605-613.1998
Specific in vivo labeling of cell surface-exposed protein loops: reactive cysteines in the predicted gating loop mark a ferrichrome binding site and a ligand-induced conformational change of the Escherichia coli FhuA protein
Abstract
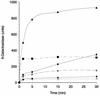
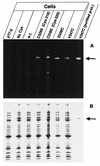
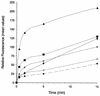
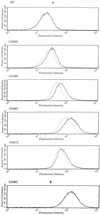
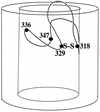
The FhuA protein of Escherichia coli K-12 transports ferrichrome, the antibiotic albomycin, colicin M, and microcin 25 across the outer membrane and serves as a receptor for the phages T1, T5, phi80, and UC-1. FhuA is activated by the electrochemical potential of the cytoplasmic membrane, which probably opens a channel in FhuA. It is thought that the proteins TonB, ExbB, and ExbD function as a coupling device between the cytoplasmic membrane and the outer membrane. Excision of 34 residues from FhuA, tentatively designated the gating loop, converts FhuA into a permanently open channel. FhuA contains two disulfide bridges, one in the gating loop and one close to the C-terminal end. Reduction of the disulfide bridges results in a low in vivo reaction of the cysteines in the gating loop and no reaction of the C-terminal cysteines with biotin-maleimide, as determined by streptavidin-beta-galactosidase bound to biotin. In this study we show that a cysteine residue introduced into the gating loop by replacement of Asp-336 displayed a rather high reactivity and was used to monitor structural changes in FhuA upon binding of ferrichrome. Flow cytometric analysis revealed fluorescence quenching by ferrichrome and albomycin of fluorescein-maleimide bound to FhuA. Ferrichrome did not inhibit Cys-336 labeling. In contrast, labeling of Cys-347, obtained by replacing Val-347 in the gating loop, was inhibited by ferrichrome, but ferrichrome quenching was negligible. It is concluded that binding of ferrichrome causes a conformational change of the gating loop and that Cys-347 is part of or close to the ferrichrome binding site. Fluorescence quenching was independent of the TonB activity. The newly introduced cysteines and the replacement of the existing cysteines by serine did not alter sensitivity of cells to the FhuA ligands tested (T5, phi80, T1, colicin M, and albomycin) and fully supported growth on ferrichrome as the sole iron source. Since cells of E. coli K-12 display no reactivity to thiol reagents, newly introduced cysteines can be used to determine surface-exposed regions of outer membrane proteins and to monitor conformational changes during their function.
Figures
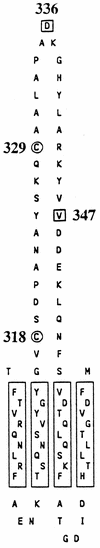

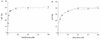
References
-
- Bardwell J C A, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. - PubMed
-
- Bayer E A, Safars M, Wilchek M. Selective labeling of sulfhydryls and disulfides on blot transfers using avidin-biotin technology: studies on purified proteins and erythrocyte membranes. Anal Biochem. 1987;161:262–271. - PubMed
-
- Bayer E A, Zalis M G, Wilchek M. 3-(N-Maleimido-propionyl) biocytin: a versatile thiol-specific biotinylating reagent. Anal Biochem. 1985;149:529–536. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

