Absence of neurological deficits following extensive demyelination in a class I-deficient murine model of multiple sclerosis
- PMID: 9461192
- PMCID: PMC5319411
- DOI: 10.1038/nm0298-187
Absence of neurological deficits following extensive demyelination in a class I-deficient murine model of multiple sclerosis
Abstract
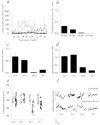
Demyelination alone has been considered sufficient for development of neurological deficits following central nervous system (CNS) disease. However, extensive demyelination is not always associated with clinical deficits in patients with multiple sclerosis (MS), the most common primary demyelinating disease in humans. We used the Theiler's murine encephalomyelitis virus model of demyelination to investigate the role of major histocompatibility complex (MHC) class I and class II gene products in the development of functional and neurophysiological deficits following demyelination. We measured spontaneous clinical activity by two independent assays and recorded hind-limb motor-evoked potentials in infected class I-deficient and class II-deficient mice of an identical genetic background as well as in highly susceptible SJL/J mice. The results show that despite a similar distribution and extent of demyelinated lesions in all mice, only class I-deficient mice were functionally normal. We propose that the mechanism by which demyelinated class I-deficient mice maintain neurologic function results from increased sodium channel densities and the relative preservation of axons. These findings are the first to implicate a role for MHC class I in the development of neurological deficits following demyelination.
Figures

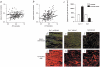

Similar articles
-
Absence of spontaneous central nervous system remyelination in class II-deficient mice infected with Theiler's virus.J Neuropathol Exp Neurol. 1999 Jan;58(1):78-91. doi: 10.1097/00005072-199901000-00009. J Neuropathol Exp Neurol. 1999. PMID: 10068316 Free PMC article.
-
Preservation of neurologic function during inflammatory demyelination correlates with axon sparing in a mouse model of multiple sclerosis.Neuroscience. 2002;111(2):399-411. doi: 10.1016/s0306-4522(02)00012-x. Neuroscience. 2002. PMID: 11983325
-
Perforin-dependent neurologic injury in a viral model of multiple sclerosis.J Neurosci. 1998 Sep 15;18(18):7306-14. doi: 10.1523/JNEUROSCI.18-18-07306.1998. J Neurosci. 1998. PMID: 9736651 Free PMC article.
-
Central nervous system demyelination and remyelination in multiple sclerosis and viral models of disease.J Neuroimmunol. 1992 Oct;40(2-3):255-63. doi: 10.1016/0165-5728(92)90141-7. J Neuroimmunol. 1992. PMID: 1430155 Review.
-
Two models of multiple sclerosis: experimental allergic encephalomyelitis (EAE) and Theiler's murine encephalomyelitis virus (TMEV) infection. A pathological and immunological comparison.Microsc Res Tech. 1995 Oct 15;32(3):215-29. doi: 10.1002/jemt.1070320305. Microsc Res Tech. 1995. PMID: 8527856 Free PMC article. Review.
Cited by
-
Th17-biased RORγt transgenic mice become susceptible to a viral model for multiple sclerosis.Brain Behav Immun. 2015 Jan;43:86-97. doi: 10.1016/j.bbi.2014.07.008. Epub 2014 Jul 18. Brain Behav Immun. 2015. PMID: 25046854 Free PMC article.
-
Animal models of multiple sclerosis: the good, the bad and the bottom line.Nat Neurosci. 2012 Jul 26;15(8):1074-7. doi: 10.1038/nn.3168. Nat Neurosci. 2012. PMID: 22837037 Free PMC article.
-
Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions.Brain Pathol. 2004 Jan;14(1):43-50. doi: 10.1111/j.1750-3639.2004.tb00496.x. Brain Pathol. 2004. PMID: 14997936 Free PMC article.
-
Remyelination-promoting human IgMs: developing a therapeutic reagent for demyelinating disease.Curr Top Microbiol Immunol. 2008;318:213-39. doi: 10.1007/978-3-540-73677-6_9. Curr Top Microbiol Immunol. 2008. PMID: 18219820 Free PMC article. Review.
-
Absence of spontaneous central nervous system remyelination in class II-deficient mice infected with Theiler's virus.J Neuropathol Exp Neurol. 1999 Jan;58(1):78-91. doi: 10.1097/00005072-199901000-00009. J Neuropathol Exp Neurol. 1999. PMID: 10068316 Free PMC article.
References
-
- Rodriguez M, Oleszak E, Leibowitz J. Theiler’s murine encephalomyelitis: A model of demyelination and persistence of virus. Crit. Rev. Immunol. 1987;7:325–365. - PubMed
-
- Pullen LC, Miller SD, Dal Canto MC, Kim BS. Class I-deficient resistant mice intracerebrally inoculated with Theiler’s virus show an increased T cell response to viral antigens and susceptibility to demyelination. Eur. J. Immunol. 1993;23:2287–2293. - PubMed
-
- Rodriguez M, et al. Abrogation of resistance to Theiler’s-induced demyelination in H–2b mice deficient in β2-microglobulin. J. Immunol. 1993;151:266–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

