An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease
- PMID: 9463404
- PMCID: PMC2212155
- DOI: 10.1084/jem.187.4.537
An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease
Abstract
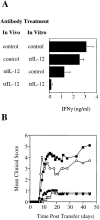
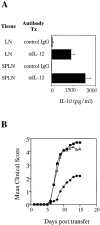
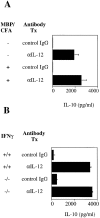
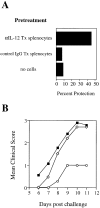
Cells of the innate immune system secrete cytokines early in immune responses that guide maturing T helper (Th) cells along appropriate lineages. This study investigates the role of cytokine networks, bridging the innate and acquired immune systems, in the pathogenesis of an organ specific autoimmune disease. Experimental allergic encephalomyelitis (EAE), a demyelinating disease of the central nervous system, is widely used as an animal model for multiple sclerosis. We demonstrate that interleukin (IL)-12 is essential for the generation of the autoreactive Th1 cells that induce EAE, both in the presence and absence of interferon gamma. The disease-promoting effects of IL-12 are antagonized by IL-10 produced by an antigen nonspecific CD4+ T cell which, in turn, is regulated by the endogenous production of IL-12. This unique immunoregulatory circuit appears to play a critical role in controlling Th cell differentiation and provides a mechanism by which microbial triggers of the innate immune system can modulate autoimmune disease.
Figures


References
-
- Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. - PubMed
-
- Sher A, Coffman RL. Regulation of autoimmunity to parasites and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. - PubMed
-
- Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. - PubMed
-
- Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. - PubMed
-
- Bergman B, Haskins K. Islet-specific T-cell clones from the NOD mouse respond to β-granule antigen. Diabetes. 1994;43:197–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

