B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5
- PMID: 9463416
- PMCID: PMC2212150
- DOI: 10.1084/jem.187.4.655
B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5
Abstract
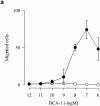
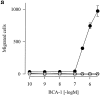
Although most leukocytes, T lymphocytes in particular, respond to several different chemokines, there is virtually no information on chemokine activities and chemokine receptors in B lymphocytes. A putative chemokine receptor, BLR1, that is expressed in Burkitt's lymphoma cells and B lymphocytes was cloned a few years ago. Deletion of the gene for BLR1 yielded mice with abnormal primary follicles and germinal centers of the spleen and Peyer's patches, reflecting the inability of B lymphocytes to migrate into B cell areas. By screening expressed sequence tag DNA sequences, we have identified a CXC chemokine, termed B cell-attracting chemokine 1 (BCA-1), that is chemotactic for human B lymphocytes. BCA-1 cDNA encodes a protein of 109 amino acids with a leader sequence of 22 residues. The mature protein shares 23-34% identical amino acids with known CXC chemokines and is constitutively expressed in secondary lymphoid organs. BCA-1 was chemically synthesized and tested for activity on murine pre-B cells 300-19 transfected with BLR1 and on human blood B lymphocytes. In transfected cells, BCA-1 induced chemotaxis and Ca2+ mobilization demonstrating that it acts via BLR1. Under the same conditions, no activity was obtained with 10 CXC and 19 CC chemokines, lymphotactin, neurotactin/fractalkine and several other peptide ligands. BCA-1 was also a highly effective attractant for human blood B lymphocytes (which express BLR1), but was inactive on freshly isolated or IL-2-stimulated T lymphocytes, monocytes, and neutrophils. In agreement with the nomenclature rules for chemokine receptors, we propose the term CXCR5 for BLR1. Together with the observed disturbance of B cell colonization in BLR1/ CXCR5-deficient mice, the present results indicate that chemotactic recruitment by locally produced BCA-1 is important for the development of B cell areas of secondary lymphoid tissues.
Figures



References
-
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. - PubMed
-
- Imai T, Yoshida T, Baba M, Nishimura M, Kakizaki M, Yoshie O. Molecular cloning of a novel T cell–directed CC chemokine expressed in thymus by signal sequence trap using Epstein-Barr virus vector. J Biol Chem. 1996;271:21514–21521. - PubMed
-
- Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, Kitaura M, Nishimura M, Kakizaki M, Nomiyama H, Yoshie O. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem. 1997;272:13803–13809. - PubMed
-
- Rossi DL, Vicari AP, Franz-Bacon K, McClanahan TK, Zlotnik A. Identification through bioinformatics of two new macrophage proinflammatory human chemokines–MIP-3α and MIP-3β. J Immunol. 1997;158:1033–1036. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

