Effects of ethanol and other alkanols on transport of acetic acid in Saccharomyces cerevisiae
- PMID: 9464405
- PMCID: PMC106099
- DOI: 10.1128/AEM.64.2.665-668.1998
Effects of ethanol and other alkanols on transport of acetic acid in Saccharomyces cerevisiae
Abstract
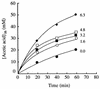
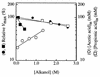
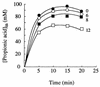
In glucose-grown cells of Saccharomyces cerevisiae IGC 4072, acetic acid enters only by simple diffusion of the undissociated acid. In these cells, ethanol and other alkanols enhanced the passive influx of labelled acetic acid. The influx of the acid followed first-order kinetics with a rate constant that increased exponentially with the alcohol concentration, and an exponential enhancement constant for each alkanol was estimated. The intracellular concentration of labelled acetic acid was also enhanced by alkanols, and the effect increased exponentially with alcohol concentration. Acetic acid is transported across the plasma membrane of acetic acid-, lactic acid-, and ethanol-grown cells by acetate-proton symports. We found that in these cells ethanol and butanol inhibited the transport of labelled acetic acid in a noncompetitive way; the maximum transport velocity decreased with alcohol concentration, while the affinity of the system for acetate was not significantly affected by the alcohol. Semilog plots of Vmax versus alcohol concentration yielded straight lines with negative slopes from which estimates of the inhibition constant for each alkanol could be obtained. The intracellular concentration of labelled acid was significantly reduced in the presence of ethanol or butanol, and the effect increased with the alcohol concentration. We postulate that the absence of an operational carrier for acetate in glucose-grown cells of S. cerevisiae, combined with the relatively high permeability of the plasma membrane for the undissociated acid and the inability of the organism to metabolize acetic acid, could be one of the reasons why this species exhibits low tolerance to acidic environments containing ethanol.
Figures

Similar articles
-
Effects of ethanol and other alkanols on passive proton influx in the yeast Saccharomyces cerevisiae.Biochim Biophys Acta. 1984 Jul 11;774(1):43-8. doi: 10.1016/0005-2736(84)90272-4. Biochim Biophys Acta. 1984. PMID: 6329295
-
Utilization of short-chain monocarboxylic acids by the yeast Torulaspora delbrueckii: specificity of the transport systems and their regulation.Biochim Biophys Acta. 1995 Jun 20;1267(2-3):122-30. doi: 10.1016/0167-4889(95)00067-3. Biochim Biophys Acta. 1995. PMID: 7612664
-
Transport of acetic acid in Zygosaccharomyces bailii: effects of ethanol and their implications on the resistance of the yeast to acidic environments.Appl Environ Microbiol. 1996 Sep;62(9):3152-7. doi: 10.1128/aem.62.9.3152-3157.1996. Appl Environ Microbiol. 1996. PMID: 8795203 Free PMC article.
-
Carboxylic Acids Plasma Membrane Transporters in Saccharomyces cerevisiae.Adv Exp Med Biol. 2016;892:229-251. doi: 10.1007/978-3-319-25304-6_9. Adv Exp Med Biol. 2016. PMID: 26721276 Review.
-
Omics analysis of acetic acid tolerance in Saccharomyces cerevisiae.World J Microbiol Biotechnol. 2017 May;33(5):94. doi: 10.1007/s11274-017-2259-9. Epub 2017 Apr 12. World J Microbiol Biotechnol. 2017. PMID: 28405910 Review.
Cited by
-
Auxotrophic Mutations Reduce Tolerance of Saccharomyces cerevisiae to Very High Levels of Ethanol Stress.Eukaryot Cell. 2015 Sep;14(9):884-97. doi: 10.1128/EC.00053-15. Epub 2015 Jun 26. Eukaryot Cell. 2015. PMID: 26116212 Free PMC article.
-
Influence of medium buffering capacity on inhibition of Saccharomyces cerevisiae growth by acetic and lactic acids.Appl Environ Microbiol. 2002 Apr;68(4):1616-23. doi: 10.1128/AEM.68.4.1616-1623.2002. Appl Environ Microbiol. 2002. PMID: 11916676 Free PMC article.
-
Mechanisms underlying lactic acid tolerance and its influence on lactic acid production in Saccharomyces cerevisiae.Microb Cell. 2021 Apr 14;8(6):111-130. doi: 10.15698/mic2021.06.751. Microb Cell. 2021. PMID: 34055965 Free PMC article. Review.
-
The Plasma Membrane at the Cornerstone Between Flexibility and Adaptability: Implications for Saccharomyces cerevisiae as a Cell Factory.Front Microbiol. 2021 Aug 9;12:715891. doi: 10.3389/fmicb.2021.715891. eCollection 2021. Front Microbiol. 2021. PMID: 34434179 Free PMC article. Review.
-
Adaptive Response and Tolerance to Acetic Acid in Saccharomyces cerevisiae and Zygosaccharomyces bailii: A Physiological Genomics Perspective.Front Microbiol. 2018 Feb 21;9:274. doi: 10.3389/fmicb.2018.00274. eCollection 2018. Front Microbiol. 2018. PMID: 29515554 Free PMC article. Review.
References
-
- Cardoso H, Leão C. Mechanisms underlying the low and high enthalpy death induced by short-chain monocarboxylic acids and ethanol in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 1992;38:388–392.
-
- Casal M, Cardoso H, Leão C. Mechanisms regulating the transport of acetic acid in Saccharomyces cerevisiae. Microbiology. 1996;142:1385–1390. - PubMed
-
- Dawson R M C, Elliot D C, William H E, Jones K M. Data for biochemical research. 3rd ed. Oxford, United Kingdom: Clarendon Press; 1989.
-
- De la Peña P, Barros F, Gascon S, Lazo P S, Ramos S. Effect of yeast killer toxin on sensitive cells of Saccharomyces cerevisiae. J Biol Chem. 1981;256:10420–10425. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

