Myelin galactolipids are essential for proper node of Ranvier formation in the CNS
- PMID: 9464989
- PMCID: PMC6792626
- DOI: 10.1523/JNEUROSCI.18-05-01642.1998
Myelin galactolipids are essential for proper node of Ranvier formation in the CNS
Abstract
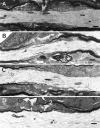
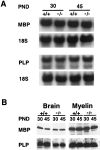
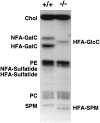
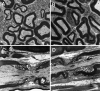
The vertebrate myelin sheath is greatly enriched in the galactolipids galactocerebroside (GalC) and sulfatide. Mice with a disruption in the gene that encodes the biosynthetic enzyme UDP-galactose:ceramide galactosyl transferase (CGT) are incapable of synthesizing these lipids yet form myelin sheaths that exhibit major and minor dense lines with spacing comparable to controls. These CGT mutant mice exhibit a severe tremor that is accompanied by hindlimb paralysis. Furthermore, electrophysiological studies reveal nerve conduction deficits in the spinal cord of these mutants. Here, using electron microscopic techniques, we demonstrate ultrastructural myelin abnormalities in the CNS that are consistent with the electrophysiological deficits. These abnormalities include altered nodal lengths, an abundance of heminodes, an absence of transverse bands, and the presence of reversed lateral loops. In contrast to the CNS, no ultrastructural abnormalities and only modest electrophysiological deficits were observed in the peripheral nervous system. Taken together, the data presented here indicate that GalC and sulfatide are essential in proper CNS node and paranode formation and that these lipids are important in ensuring proper axo-oligodendrocyte interactions.
Figures



References
-
- Benjamins JA, Studzinski DM, Skoff RP. Analysis of myelin proteolipid protein and F0 ATPase subunit 9 in normal and jimpy CNS. Neurochem Res. 1994;19:1013–1022. - PubMed
-
- Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, Popko B. Myelination in the absence of galactocerebroside and sulfatide: normal structure and abnormal function and regional instability. Cell. 1996;86:209–219. - PubMed
-
- Dentinger MP, Barron KD, Csiza CK. Ultrastructure of the central nervous system in a myelin deficient rat. J Neurocytol. 1982;11:671–691. - PubMed
-
- Duncan ID, Griffiths IR, Munz M. “Shaking pup”: a disorder of central myelination in the spaniel dog. III. Quantitative aspects of the glia and myelin in the spinal cord and optic nerve. Neuropathol Appl Neurobiol. 1983;9:355–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
